
Exhibit 99.1

Expanding the Potential of CAR T CellTherapy NASDAQ:XBIO xeneticbio.com InvestorPresentation September 2021

Forward-LookingStatements Thispresentationcontainsforward-lookingstatementsthatweintendtobesubjecttothesafeharborprovisionsofthePrivateSecuritiesLitigationReform Act of1995.Allstatementscontainedinthispresentationotherthanstatementsofhistoricalfactsmayconstituteforward-lookingstatementswithinthe meaning ofthefederalsecuritieslaws.Thesestatementscanbeidentifiedbywordssuchas"expects,""plans,""projects,""will,""may,""anticipates,""believes," "should,""intends,""estimates,"andotherwordsofsimilarmeaning,including,butnotlimitedto,allstatementsregarding:theCARTfocusandpotential upside with PolyXentechnology set forthunder the “InvestmentHighlights” section of this presentation; XCARTopportunities, including targeting tumor-specificantigensthatareindependentofCD19orotherantigenscommontoallB-CellsandadvancingtowardsaPhase1study;plans toleverage outsourcedrelationships;potentialforXCARTtoresultinincreasedefficacy,safetyandtolerabilityovercurrentlyapprovedCARTtherapies;thepotentialto conductaPhase1trialwithacademiccollaborators; potentialutilitiesofPolyXen;andexpectationsregardingcashrunwayfundingtheCompanythroughan INDfiling;aswellasallstatementssetforthunderthe“DrivingDevelopmentThroughOutsourcedRelationships”sectionofthispresentation,includingthose relatedtoupcomingpotentialmilestones,allstatementssetforthunderthe“InvestmentSummary”sectionofthispresentation;all statementsregarding expectationsthattheCARTtherapieswillholdsignificantrevenueshareby2026,includinganticipationsthattherewillbeasignificantdropinRituxanuse. Anyforward-lookingstatementscontainedhereinarebasedoncurrentexpectationsandaresubjecttoanumberofrisksanduncertainties.Manyfactors couldcauseouractualactivitiesorresultstodiffermateriallyfromtheactivitiesandresultsanticipatedinforward-lookingstatements.Importantfactorsthat couldcauseactualresultstodiffermateriallyfromsuchplans,estimatesorexpectationsinclude,amongothers,(1)unexpectedcosts,chargesorexpenses resultingfromtheacquisitionoftheCARTtechnology;(2)uncertaintyoftheexpectedfinancialperformanceoftheCompany;(3)failuretorealizethe anticipatedpotentialoftheXCARTtechnology;(4)theabilityoftheCompanytoimplementitsbusinessstrategy;(5)failureofScrippsResearchand/or PharmsynthezortheotheracademicinstitutionsinBelarusandRussia(asapplicable)toperformtheirobligationsundertheirrespectiveagreements; (6) failureoftheCompanyandPharmsyntheztoreachagreementswiththecontractsitesontermsfavorabletotheCompany,oratall;(7)failureofourlicensees tosuccessfullyutilizethePolyXentechnologyandgenerateroyaltiesfortheCompany;and(8)otherriskfactorsasdetailedfromtimetotimeinthe Company’sreportsfiledwiththeSEC,includingitsannualreportonForm10-K,periodicquarterlyreportsonForm10-Q,periodiccurrentreportsonForm8-K andotherdocumentsfiledwiththeSEC.Theforegoinglistofimportantfactorsisnotexclusive.Inaddition,forward-lookingstatementsmayalsobeadversely affectedbygeneralmarketfactors,generalbusinessandeconomicconditions,includingpotentialadverseeffectsofpublic healthissuessuchastheCOVID-19 pandemic,competitiveproductdevelopment,productavailability,federalandstateregulationsandlegislation,theregulatoryprocessfornewproduct candidatesandindications,manufacturingissuesthatmayarise,patentpositionsandlitigation,amongotherfactors.Theforward-looking statements containedinthispresentationspeakonlyasofthedatethestatementsweremade,andtheCompanydoesnotundertakeanyobligationtoupdateforward- lookingstatements,exceptasrequiredbylaw. Disclaimer Theinformationcontainedinthispresentationisprovidedforinformationalanddiscussionpurposesonlyandisnot,andmaynotbereliedoninanymanner aslegal,business,financial,taxorinvestmentadviceorasanoffertosellorasolicitationofanoffertobuyaninterestinXeneticBiosciences,Inc.orto participateinanytradingstrategy. 2
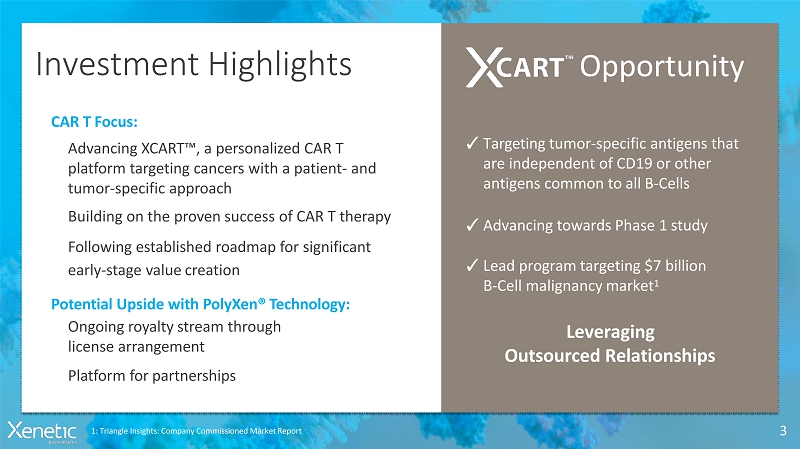
InvestmentHighlights 3 Opportunity ? Targeting tumor-specific antigens that are independent of CD19 or other antigens common to allB-Cells ? Advancing towards Phase 1study ? Lead program targeting $7 billion B-Cell malignancymarket 1 Leveraging OutsourcedRelationships 1: Triangle Insights: Company Commissioned MarketReport CAR TFocus: Advancing XCART , a personalized CAR T platform targeting cancers with a patient-and tumor-specificapproach Building on the proven success of CAR T therapy Following established roadmap forsignificant early-stage valuecreation Potential Upside with PolyXen®Technology: Ongoing royalty stream through licensearrangement Platform forpartnerships

Team with ProvenExpertise Jeffrey F.Eisenberg Chief Executive Officer &Director Life Sciences executive with over 20 years of successful track record in value creation in both private and public companies; former CEO of Noven Pharmaceuticals, responsible for leading 2 product launches and Noven’s Novogyne Women’s Health joint venture withNovartis Curtis Lockshin,Ph.D. Chief ScientificOfficer 20 years Biotech/Pharma management experience, including discovery, preclinical and clinical development and commercial manufacturing; former CEO of SciVac Therapeutics, CTO of VBI Vaccines and VP of Corporate R&D Initiatives for OPKOHealth James F. Parslow, MBA,CPA Chief FinancialOfficer Over 30 years of experience providing financial and business leadership to biotech, manufacturing, technology, business-to-business e-commerce and cleantechindustries 4

Scientific Advisory Board withExtensive Cell Therapy Development Experience 5 Dr. MatthewFrigault Medical Oncologist in the Hematologic Malignancy Program at theMassachusetts General Hospital Cancer Center, as well as Assistant Director of the Cellular Immunotherapy Program; serves as Instructor at Harvard MedicalSchool Dr. AlexanderGabibov HeadoftheShemyakin&OvchinnikovInstituteofBioorganicChemistryatthe Russian Academy ofScience Dr. GuentherKoehne Internationally recognized cancer specialist and current Chief of Blood &Marrow Transplant and Hematologic Oncology at the Miami CancerInstitute Dr. GregMacMichael PresidentandFounderofCMCBioServices,LLC;PreviouslyservedastheSeniorVP of Technical Operations at Axovant Gene Therapies; VP of Development, Manufacturing and Quality Control at NantKwest Therapeutics; and Senior VP of Process, Development, Manufacturing and Quality Assurance at RocketPharma Dr. MaksimMamonkin Assistant Professor, Pathology and Immunology and an independent faculty member at the Center for Cell and Gene Therapy at Baylor College ofMedicine Dr. JiaXie Assistant professor at University of Miami Department of Chemistry, assistant professorofPsychiatryandBehaviorScienceatUniversityofMiamiMillerSchoolof Medicine; and visiting investigator at the Department of Chemistry at Scripps ResearchInstitute Dr. Alexey V.Stepanov Senior Staff Scientist in the Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry; Senior Staff Scientist position in the Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology in Russia; Professional scientific collaborator of Dr. Richard Lerner's laboratory in The Scripps ResearchInstitute

Personalized CAR T platform targetingcancers with a patient-and tumor-specificapproach 6 Platform
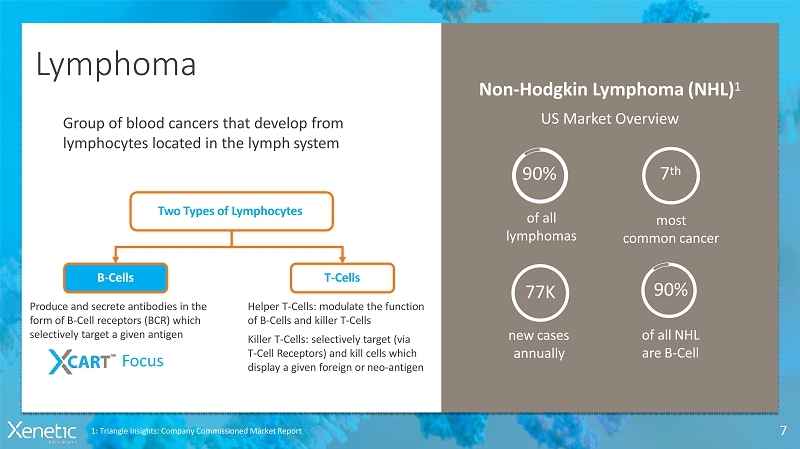
Lymphoma 7 Group of blood cancers that developfrom lymphocytes located in the lymphsystem Two Types ofLymphocytes B-Cells T-Cells Produce and secrete antibodies in the form of B-Cell receptors (BCR) which selectively target a givenantigen Helper T-Cells: modulate the function of B-Cells and killerT-Cells Killer T-Cells: selectively target(via T-Cell Receptors) and kill cellswhich display a given foreign orneo-antigen Focus Non-Hodgkin Lymphoma(NHL) 1 US MarketOverview of all lymphomas 90% most commoncancer 7 th newcases annually 77K of allNHL areB-Cell 90% 1: Triangle Insights: Company Commissioned MarketReport
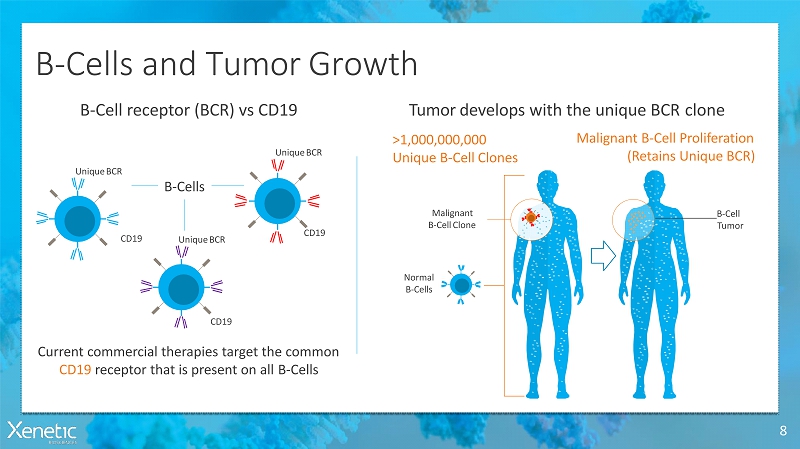
B-Cells and TumorGrowth 8 Tumor develops with the unique BCRcloneB-Cell receptor (BCR) vsCD19 Current commercial therapies target the common CD19 receptor that is present on allB-Cells CD19 CD19 Normal B-Cells Malignant B-Cell Proliferation (Retains UniqueBCR) >1,000,000,000 Unique B-CellClones CD19 UniqueBCR UniqueBCR UniqueBCR B-Cells Malignant B-CellClone B-Cell Tumor
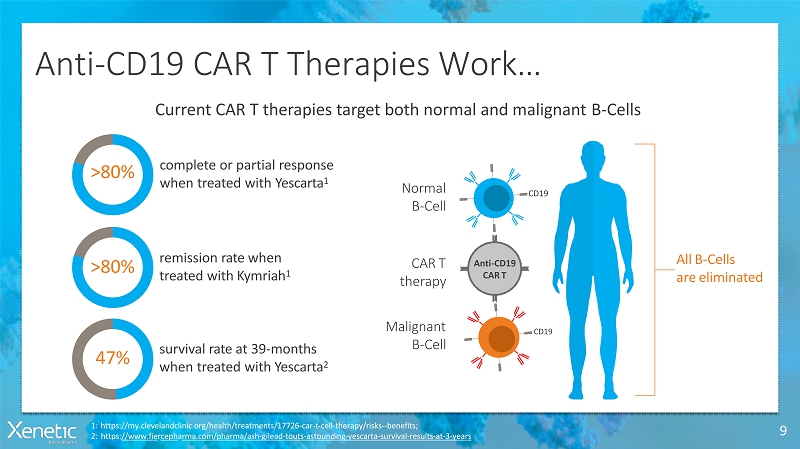
Anti-CD19 CAR T TherapiesWork… 9 1:https://my.clevelandclinic.org/health/treatments/17726-car-t-cell-therapy/risks--benefits; 2:https://www.fiercepharma.com/pharma/ash-gilead-touts-astounding-yescarta-survival-results-at-3-years Current CAR T therapies target both normal and malignantB-Cells >80% complete or partialresponse when treated withYescarta 1 >80% remission rate when treated withKymriah 1 47% survival rate at 39-months when treated withYescarta 2 AllB-Cells areeliminated Anti-CD19 CART CART therapy Normal B-Cell Malignant B-Cell CD19 CD19
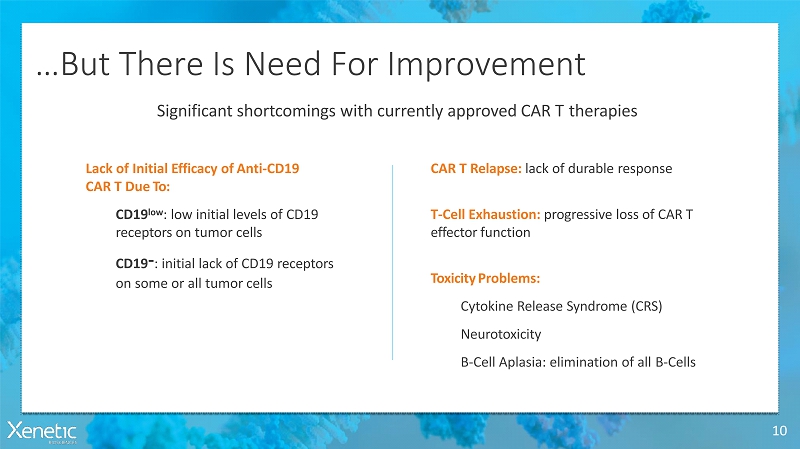
…But There Is Need ForImprovement 10 CAR T Relapse: lack of durableresponse T-Cell Exhaustion: progressive loss of CART effectorfunction ToxicityProblems: Cytokine Release Syndrome(CRS) Neurotoxicity B-Cell Aplasia: elimination of allB-Cells Significant shortcomings with currently approved CAR Ttherapies Lack of Initial Efficacy ofAnti-CD19 CAR T DueTo: CD19 low : low initial levels ofCD19 receptors on tumorcells CD19 - : initial lack of CD19 receptors on some or all tumorcells
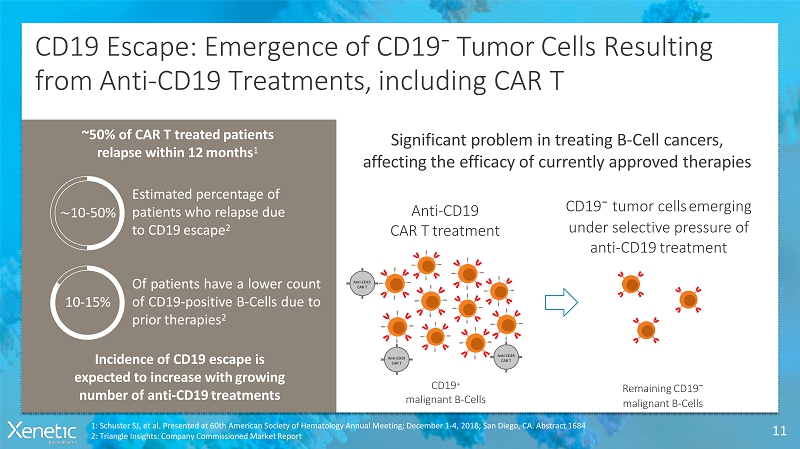
CD19 Escape: Emergence of CD19 - Tumor CellsResulting from Anti-CD19 Treatments, including CART 11 Estimated percentage of patients who relapse due to CD19escape 2 ~10-50% Ofpatientshavealowercount ofCD19-positiveB-Cellsdueto priortherapies 2 10-15% Incidence of CD19 escape is expected to increase withgrowing number of anti-CD19treatments Significant problem in treating B-Cellcancers, affecting the efficacy of currently approvedtherapies ~50% of CAR T treatedpatients relapse within 12months 1 Anti-CD19 CAR Ttreatment CD19 - tumor cellsemerging under selective pressure of anti-CD19treatment 1: Schuster SJ, et al. Presented at 60th American Society of Hematology Annual Meeting; December 1-4, 2018; San Diego, CA. Abstract 1684 2: Triangle Insights: Company Commissioned MarketReport CD19 + malignantB-Cells RemainingCD19 - malignantB-Cells Anti-CD19 CART Anti-CD19 CART Anti-CD19 CART
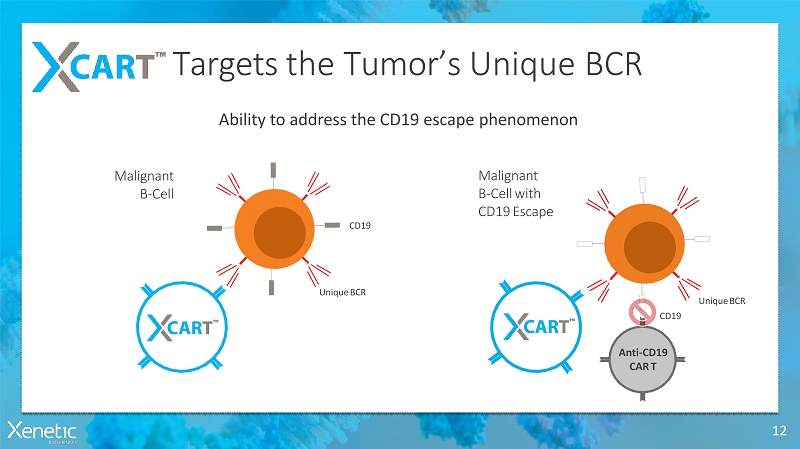
Targets the Tumor’s UniqueBCR 12 Ability to address the CD19 escapephenomenon Malignant B-Cell Malignant B-Cellwith CD19Escape UniqueBCR CD19 UniqueBCR CD19 Anti-CD19 CART
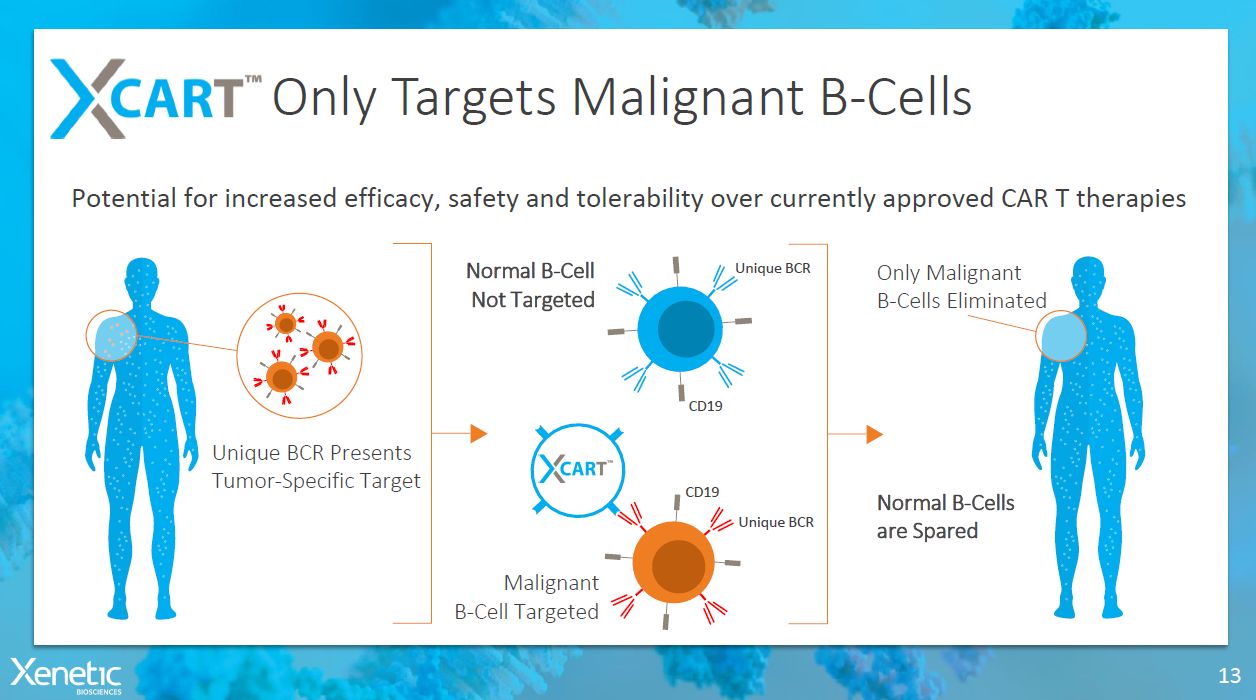
Only Targets MalignantB-Cells Potential for increased efficacy, safety and tolerability over currently approved CAR Ttherapies Malignant B-CellTargeted 13 Unique BCR Presents Tumor-SpecificTarget OnlyMalignant B-CellsEliminated NormalB-Cells areSpared CD19 CD19 UniqueBCR UniqueBCR NormalB-Cell NotTargeted
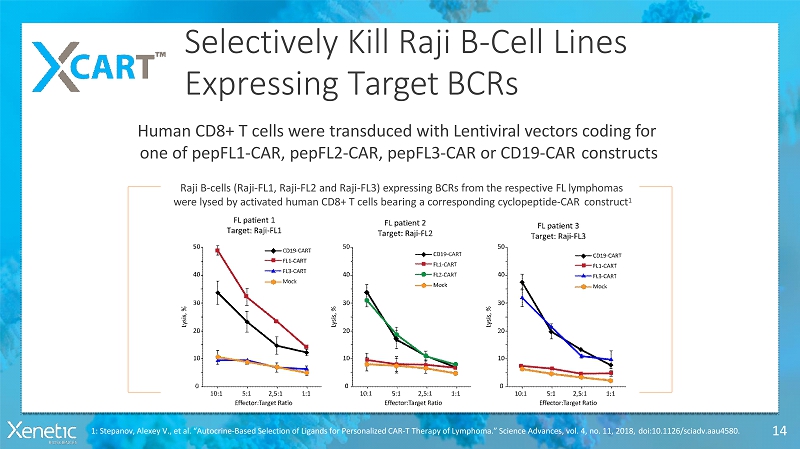
Selectively Kill Raji B-CellLines Expressing TargetBCRs 14 Raji B-cells (Raji-FL1, Raji-FL2 and Raji-FL3) expressing BCRs from the respective FLlymphomas were lysed by activated human CD8+ T cells bearing a corresponding cyclopeptide-CARconstruct 1 Human CD8+ T cells were transduced with Lentiviral vectors coding for one of pepFL1-CAR, pepFL2-CAR, pepFL3-CAR or CD19-CARconstructs 1: Stepanov, Alexey V., et al. “Autocrine-Based Selection of Ligands for Personalized CAR-T Therapy of Lymphoma.” Science Advances, vol. 4, no. 11, 2018,doi:10.1126/sciadv.aau4580.
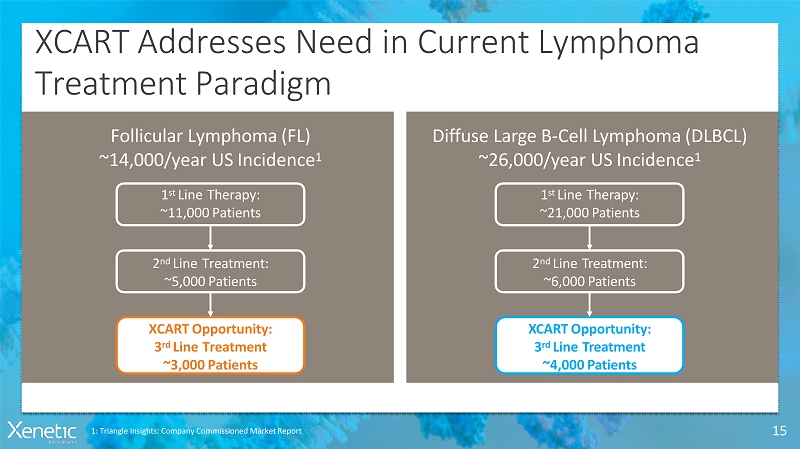
XCART Addresses Need in CurrentLymphoma TreatmentParadigm 15 Follicular Lymphoma(FL) ~14,000/year USIncidence 1 1 st LineTherapy: ~11,000Patients 2 nd LineTreatment: ~5,000Patients XCARTOpportunity: 3 rd LineTreatment ~3,000Patients Diffuse Large B-Cell Lymphoma(DLBCL) ~26,000/year USIncidence 1 1 st LineTherapy: ~21,000Patients 2 nd LineTreatment: ~6,000Patients XCARTOpportunity: 3 rd LineTreatment ~4,000Patients 1: Triangle Insights: Company Commissioned MarketReport

Leveraging OutsourcedRelationships Expediting Development Pipeline with Proven Expertise andCapabilities 16
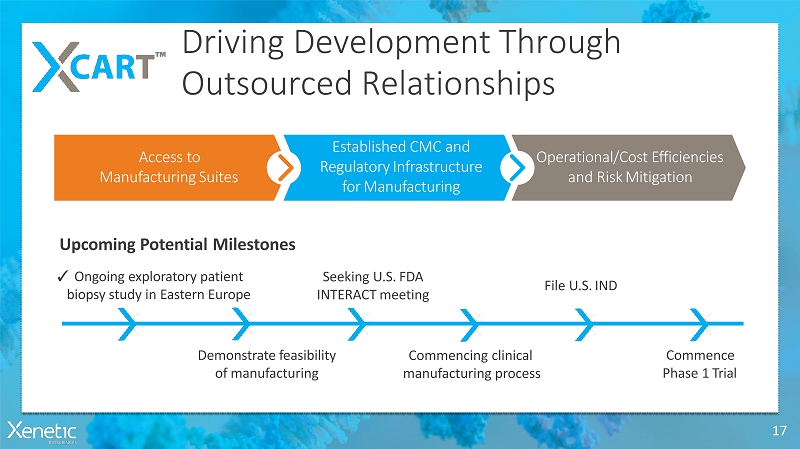
Driving DevelopmentThrough OutsourcedRelationships 17 Commencing clinical manufacturingprocess ? Ongoing exploratorypatient biopsy study in EasternEurope Seeking U.S.FDA INTERACTmeeting File U.S.IND Commence Phase 1Trial Upcoming PotentialMilestones Access to ManufacturingSuites Established CMC and RegulatoryInfrastructure forManufacturing Operational/Cost Efficiencies and RiskMitigation Demonstratefeasibility ofmanufacturing
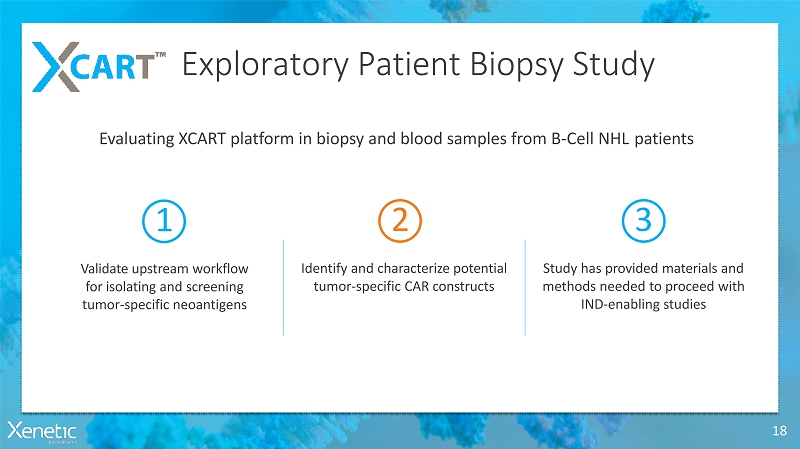
Exploratory Patient BiopsyStudy 18 Evaluating XCART platform in biopsy and blood samples from B-Cell NHLpatients 1 Validate upstream workflow for isolating and screening tumor-specificneoantigens 2 Identify and characterize potential tumor-specific CARconstructs 3 Study has provided materials and methods needed to proceed with IND-enablingstudies
Collaborators 19 Additional collaborations advancing XCART toward IND-enablingstudies AcademicCollaborators (One of the original developers of the XCARTplatform) Design and implementation of the preclinical developmentprogram Method development activities supporting process development for clinicalmanufacturing Working with world-renowned academic institutions, researchers and clinicalinvestigators Access to methods and materials, including clinical samples, for optimizing the overall XCART workflow OutsourcedRelationships Leveraging additional vendors to expedite commercialdevelopment Developing cost-effective clinical manufacturing process for patient- specific cell therapyproducts
AcademicCollaborator 20 PHARMSYNTHEZ Research organization coordinating activities with partnered academic institutions in EasternEurope Supports optimization of overall XCARTworkflow Access to clinical centers and B-Cell non-Hodgkin lymphoma (NHL)patients Potential to conduct Phase 1trial P1

PolyXen® PSA TechnologyPlatform Enables Next Generation BiologicDrugs 21
PolyXen: Next GenerationHalf-Life Extension PlatformTechnology 22 Polysialylation employs the biological polymer polysialic acid (PSA) to modulate the PK and PD profiles of proteindrugs Clinically demonstrated to extend half-life of therapeuticproteins Applicable to franchise extensionsas well as candidates indevelopment ? Potential utility in other molecule classes such as peptides and smallmolecules Receiving royalties on net sales through licensing arrangement in the field of blood coagulation disorders Pharmsynthez filed registration dossier in Russia for Epolong, a polysialylated form of recombinant human erythropoietin as a treatment for anemia in patients with chronic kidney disease Generating Royalty Stream Platform for Partnerships Extensive IPprotection
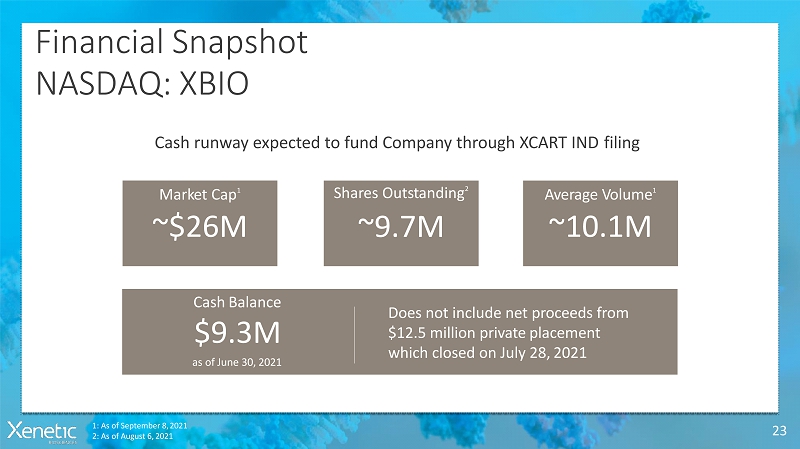
FinancialSnapshot NASDAQ:XBIO 23 Cash runway expected to fund Company through XCART INDfiling SharesOutstanding 2 ~9.7M Market Cap 1 ~$26M AverageVolume 1 ~10.1M 1: As of September 8,2021 2: As of August 6,2021 CashBalance $9.3M as of June 30,2021 Does not include net proceedsfrom $12.5 million privateplacement which closed on July 28,2021
InvestmentSummary 24 Advancing XCART program throughpreclinical development into the clinic as quickly aspossible Positioning to have a transformative impact in the CAR Tspace $7B 1: Triangle Insights: Company Commissioned MarketReport PolyXen Truly differentiated CAR Ttechnology Lead program targeting growing $7 billion B-Cell malignancymarket 1 Upside through licensingarrangements Strong balance sheet expected to fund Company through INDfiling

Expanding the Potential of CAR T CellTherapy NASDAQ:XBIO xeneticbio.com JTC Team,LLC. T:833-475-8247 xbio@jtcir.com

Appendix 26
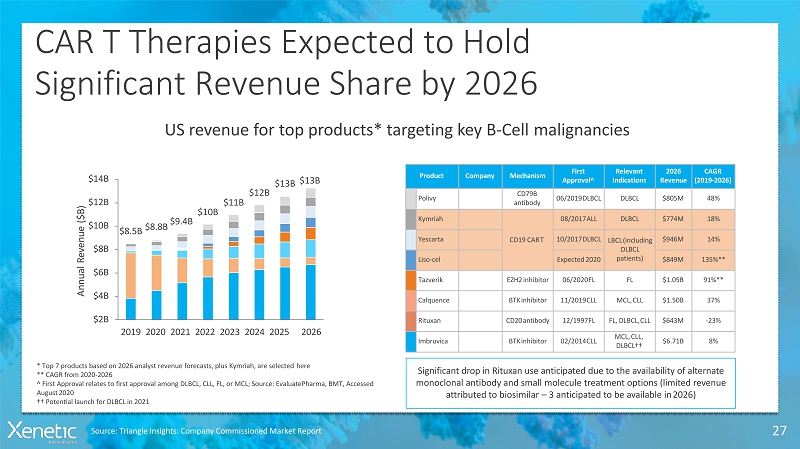
CAR T Therapies Expected toHold Significant Revenue Share by 2026 27 US revenue for top products* targeting key B-Cellmalignancies Product Company Mechanism First Approval^ Relevant Indications 2026 Revenue CAGR (2019-2026) Polivy CD79B antibody 06/2019DLBCL DLBCL $805M 48% Kymriah CD19 CART 08/2017ALL DLBCL $774M 18% Yescarta 10/2017DLBCL LBCL(including DLBCL patients) $946M 14% Liso-cel Expected2020 $849M 135%** Tazverik EZH2inhibitor 06/2020FL FL $1.05B 91%** Calquence BTKinhibitor 11/2019CLL MCL,CLL $1.50B 37% Rituxan CD20antibody 12/1997FL FL, DLBCL,CLL $643M -23% Imbruvica BTKinhibitor 02/2014CLL MCL,CLL, DLBCL†† $6.71B 8% Significant drop in Rituxan use anticipated due to the availability of alternate monoclonal antibody and small molecule treatment options (limited revenue attributed to biosimilar –3 anticipated to be available in2026) $8.5B $8.8B $9.4B $10B $11B $12B $13B $13B $10B $12B $14B $8B $6B $4B $2B 2019 2020 2021 2022 2023 2024 2025 2026 A n n u a l R e v e n u e ( $ B ) * Top 7 products based on 2026 analyst revenue forecasts, plus Kymriah, are selectedhere ** CAGR from2020-2026 ^ First Approval relates to first approval among DLBCL, CLL, FL, or MCL; Source: EvaluatePharma, BMT, Accessed August2020 †† Potential launch for DLBCL in2021 Source: Triangle Insights: Company Commissioned MarketReport
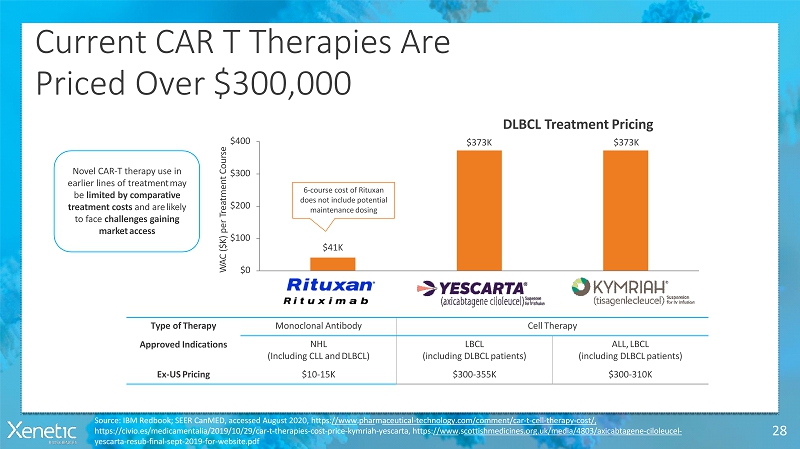
Current CAR T TherapiesAre Priced Over$300,000 28 $41K $373K DLBCL TreatmentPricing $373K $0 $100 $200 $300 $400 W A C ( $ K ) p e r T r e a t m e n t C o u r s e Type ofTherapy MonoclonalAntibody CellTherapy ApprovedIndications NHL (Including CLL andDLBCL) LBCL (including DLBCLpatients) ALL,LBCL (including DLBCLpatients) Ex-USPricing $10-15K $300-355K $300-310K Novel CAR-T therapy use in earlier lines of treatmentmay be limited by comparative treatment costs and arelikely to face challenges gaining marketaccess 6-course cost of Rituxan does not includepotential maintenancedosing Source: IBM Redbook; SEER CanMED, accessed August 2020, https://www.pharmaceutical-technology.com/comment/car-t-cell-therapy-cost/, https://civio.es/medicamentalia/2019/10/29/car-t-therapies-cost-price-kymriah-yescarta, https://www.scottishmedicines.org.uk/media/4803/axicabtagene-ciloleucel- yescarta-resub-final-sept-2019-for-website.pdf