

Enhancing lives with transformative therapies www.xeneticbio.com NASDAQ: XBIO Corporate Presentation June 2019 Filed by Xenetic Biosciences, Inc. pursuant to Rule 425 under the Securities Act of 1933, as amended, and deemed filed pursuant to Rule 14a - 6 under the Securities Exchange Act of 1934, as amended Filer: Xenetic Biosciences, Inc. Subject Company: Xenetic Biosciences, Inc. Commission File No.: 001 - 37937 Date: June 18, 2019

Forward - Looking Statements This presentation contains forward - looking statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 . All statements contained in this presentation other than statements of historical facts may constitute forward - looking statements within the meaning of the federal securities laws . These statements can be identified by words such as "expects," "plans," "projects," "will," "may," "anticipates," "believes," "should," "intends," "estimates," and other words of similar meaning . Any forward - looking statements contained herein are based on current expectations, and are subject to a number of risks and uncertainties . These forward - looking statements include, but are not limited to, statements regarding the acquisition and development of the CAR T technology, such as the anticipated effects of the acquisition on the Company’s position in the development of new oncology therapeutics, the expected leveraging opportunities resulting from the acquisition, the expected results of the XCART technology, and the Company’s future plans for the XCART clinical program and development efforts in the area of CAR T therapy after the acquisition is consummated . Many factors could cause our actual activities or results to differ materially from the activities and results anticipated in forward - looking statements . Important factors that could cause actual results to differ materially from such plans, estimates or expectations include, among others, ( 1 ) that one or more closing conditions to the acquisition of the CAR T technology, including certain regulatory approvals, may not be satisfied or waived, on a timely basis or otherwise, or that the required approval by the stockholders of the Company may not be obtained ; ( 2 ) the condition that the Company have adequate financing to fund its future working capital obligations may not be met ; ( 3 ) the risk that the acquisition may not be completed on the terms or in the time frame expected by the Company, or at all ; ( 4 ) unexpected costs, charges or expenses resulting from the acquisition ; ( 5 ) uncertainty of the expected financial performance of the Company following completion of the acquisition ; ( 6 ) failure to realize the anticipated benefits of the acquisition ; ( 7 ) the ability of the Company to implement its business strategy ; ( 8 ) the occurrence of any event that could give rise to termination of the acquisition ; and ( 9 ) other risk factors as detailed from time to time in the Company’s reports filed with the SEC, including its annual report on Form 10 - K, periodic quarterly reports on Form 10 - Q, periodic current reports on Form 8 - K and other documents filed with the SEC . In addition, forward - looking statements may also be adversely affected by general market factors, competitive product development, product availability, federal and state regulations and legislation, the regulatory process for new product candidates and indications, manufacturing issues that may arise, patent positions and litigation, among other factors . The forward - looking statements contained in this presentation speak only as of the date the statements were made, and the Company does not undertake any obligation to update forward - looking statements, except as required by law . 2

Forward - Looking Statements ADDITIONAL INFORMATION AND WHERE TO FIND IT In connection with the acquisition, the Company has filed and had declared effective with the Securities and Exchange Commission (the “SEC”), a registration statement on Form S - 4 that includes a combined definitive proxy statement/prospectus . This communication is not a substitute for any proxy statement, prospectus registration statement, or other documents the Company may file with the SEC in connection with the acquisition . INVESTORS AND SECURITY HOLDERS ARE URGED TO READ CAREFULLY AND IN THEIR ENTIRETY THESE DOCUMENTS, ANY AMENDMENTS OR SUPPLEMENTS TO THESE DOCUMENTS, AND OTHER DOCUMENTS FILED BY THE COMPANY WITH THE SEC IN CONNECTION WITH THE ACQUISITION, BECAUSE THESE DOCUMENTS CONTAIN IMPORTANT INFORMATION . Investors and security holders will be able to obtain free copies of these materials and other documents filed with the SEC by the Company through the website maintained by the SEC at www . sec . gov . Investors and security holders may also obtain free copies of the documents filed by the Company with the SEC by directing a written request to Xenetic Biosciences, Inc . , 40 Speen Street, Suite 102 , Framingham, MA 01701 or by calling 781 - 778 - 7720 . PARTICIPANTS IN THE SOLICITATION This communication is not a solicitation of a proxy from any investor or security holder . The Company, its respective directors, executive officers and other members of its management and employees may be deemed to be participants in the solicitation of proxies from shareholders of the Company in connection with the acquisition . Information regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of proxies in connection with the acquisition, including a description of their direct or indirect interests, by security holdings or otherwise, will be set forth in the relevant materials when filed with the SEC . Information regarding the directors and executive officers of the Company is contained in its Annual Report on Form 10 - K for the year ended December 31 , 2018 , which was filed with the SEC on March 29 , 2019 as amended on April 30 , 2019 , and its Registration Statement on Form S - 4 including a combined proxy statement/prospectus, which was filed on March 29 , 2019 , as amended and declared effective on May 22 , 2019 . These documents can be obtained free of charge from the sources indicated above . 3
Xenetic Investment Highlights 4 1: The acquisition of 1) Hesperix , which owned rights to the XCART platform based on the assignment agreement discussed in greater detail in a Form 8 - K filed wit h the SEC on March 4, 2019 as amended, and 2) an exclusive license of the rights to the XCART platform owned by Scripps Research Institute obtained through an assignment agreement discussed in greater detail in a For m 8 - K filed with the SEC on March 4, 2019 as amended, are subject to closing; 2: Market Reports World. GLOBAL NON - HODGKIN LYMPHOMA THERAPEUTICS MARKET - SEGMENTED BY TYPE OF TREATMENT - GROWTH, TRENDS AND FORECASTS (2018 - 2023); BioPharm Insight Surveillance, Epidemiology, and End Results (SEER) 9 registries, National Cancer Institute, 2017 Transformative acquisition of the XCART platform positions Xenetic to address high value oncology market XCART Platform 1 Expanding the Potential of CAR T Cell Therapy • Proof - of - mechanism and preclinical evidence of target specificity • Pursuing academic collaboration for early program development • Over $5 billion initial market opportunity in B - cell non - Hodgkin lymphoma 2

Experienced Management Team 5 Jeffrey F. Eisenberg Chief Executive Officer & Director Life Sciences executive with over 20 years of successful track record in value creation in both private and public companies; former CEO of Noven Pharmaceuticals and responsible for 2 product launches and led Noven’s Novogyne Women’s Health joint venture with Novartis Curtis Lockshin, Ph.D. Chief Scientific Officer 20 years Biotech/Pharma management experience, including discovery, preclinical and clinical development and commercial manufacturing; former CEO of SciVac Therapeutics, CTO of VBI Vaccines and VP of Corporate R&D Initiatives for OPKO Health James F. Parslow, MBA, CPA Chief Financial Officer Over 30 years of experience providing financial and business leadership to biotech, manufacturing, technology, business - to - business e - commerce and cleantech industries

Board of Directors 6 Adam Logal Chairman CFO, OPKO Health; Former Director, VBI Vaccines; Nabi Biopharmaceuticals Dmitry Genkin Director Former Head of Pharmavit , one of Russia’s largest pharmaceutical companies; Chairman, PJSC Pharmsynthez James Eric Callaway, Ph.D. Director Seasoned CEO within the venture - backed community and current CEO of Kalgene ; Former Head of Development, Elan Pharmaceuticals Roman Knyazev Director Senior Investment Manager, Rusnano; Chairman, Pharmsynthez , PETAR and Nanolek ; Director, SynBio Firdaus Jal Dastoor, FCS Director Fellow Member of The Institute of Company Secretaries of India; Group Director of the Poonawalla Group of Companies Roger Kornberg, Ph.D. Director Winzer Professor of Medicine in the Department of Structural Biology at Stanford University; Nobel Prize in Chemistry - Molecular Basis of Eukaryotic Transcription Jeffrey F. Eisenberg Chief Executive Officer & Director
Scientific Advisory Board Dr. Matthew Frigault Medical Oncologist in the Hematologic Malignancy Program at the Massachusetts General Hospital Cancer Center, as well as Assistant Director of the Cellular Immunotherapy Program, serves as Instructor at Harvard Medical School. Prof. Dr. Franco Cavalli Former Scientific Director, Institute of Oncology of Southern Switzerland (IOSI), Head of Organizing Committee of International Conference on Malignant Lymphoma (ICML), Chairman of Scientific Committee of the European School of Oncology (ESO) and of the World Oncology Forum (WOF), Founder of the International Extranodal Lymphoma Study Group (IELSG). Dr. Alexander Gabibov Head of the Shemyakin & Ovchinnikov Institute of Bioorganic Chemistry at the Russian Academy of Science. Dr. Gabibov holds several senior positions in the Biochemistry sphere in both Russia and France. In 2008, he was appointed President of the Russian Biochemical and Molecular Biology Society. In 2009, Dr. Gabibov took up the role of Foreign Correspondent at the National Academy of Pharmacy in France. Dr. Guenther Koehne Internationally recognized cancer specialist and current Chief of Blood & Marrow Transplant and Hematologic Oncology at the Miami Cancer Institute; noteworthy reputation for his work in adoptive immunotherapeutic approaches with antigen - specific, donor - derived T lymphocytes in the treatment of viral complications following allogeneic transplants and has developed new approaches to the treatment of patients with high - risk multiple myeloma. Dr. Davide Rossi Deputy Head of the Division of Hematology and co - chair of the Clinical Lymphoid Tumors Investigation Program (CLIP) at the Institute of Oncology of Southern Switzerland (IOSI), Head of the Experimental Hematology research program at the Institute of Oncology Research (IOR), Member of Organizing Committee of the International Conference on Malignant Lymphoma. Dr. Rossi’s translational research focuses on lymphomas and chronic lymphocytic leukemia. 7

XCART Platform 1 Expanding the Potential of CAR T Cell Therapy 1: Acquisition, subject to the satisfaction of the closing conditions, is expected to close in the first half of 2019. Please see our Registration Statement on Form S - 4 for more details
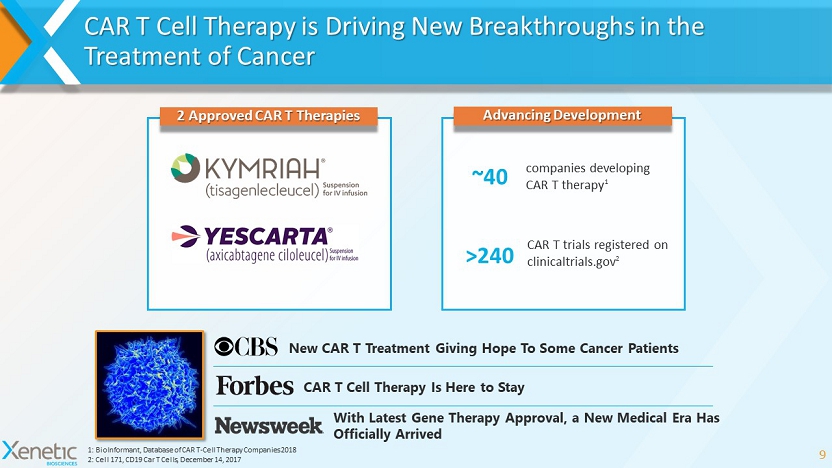
CAR T Cell Therapy is Driving New Breakthroughs in the Treatment of Cancer 9 New CAR T Treatment Giving Hope To Some Cancer Patients CAR T Cell Therapy Is Here to Stay With Latest Gene Therapy Approval, a New Medical Era Has Officially Arrived 1: BioInformant , Database of CAR T - Cell Therapy Companies 2018 2: Cell 171, CD19 Car T Cells, December 14, 2017 2 Approved CAR T Therapies companies developing CAR T therapy 1 Advancing Development ~40 CAR T trials registered on clinicaltrials.gov 2 >240
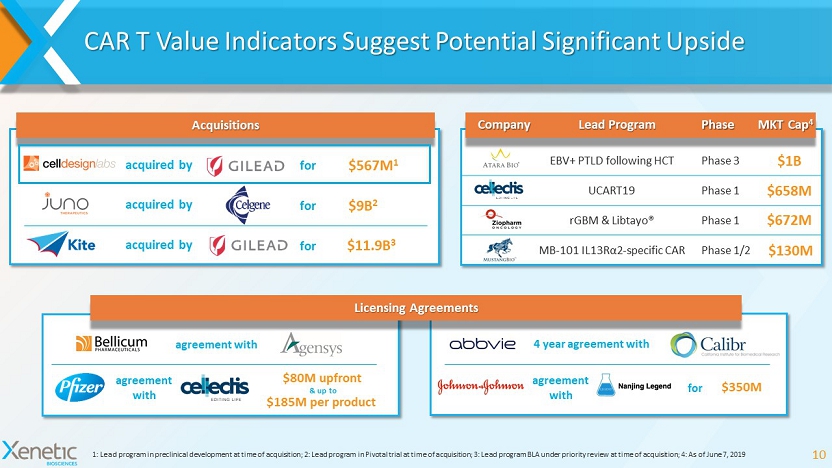
CAR T Value Indicators Suggest Potential Significant Upside 10 1: Lead program in preclinical development at time of acquisition; 2: Lead program in Pivotal trial at time of acquisition; 3 : L ead program BLA under priority review at time of acquisition; 4: As of June 7, 2019 EBV+ PTLD following HCT Phase 3 $1B UCART19 Phase 1 $658M rGBM & Libtayo ® Phase 1 $672M MB - 101 IL13R α2 - specific CAR Phase 1/2 $130M $9B 2 $567M 1 acquired by for acquired by for Acquisitions Company Lead Program Phase MKT Cap 4 agreement with agreement with $80M upfront $185M per product & up to 4 year agreement with agreement with $350M for Licensing Agreements $11.9B 3 acquired by for
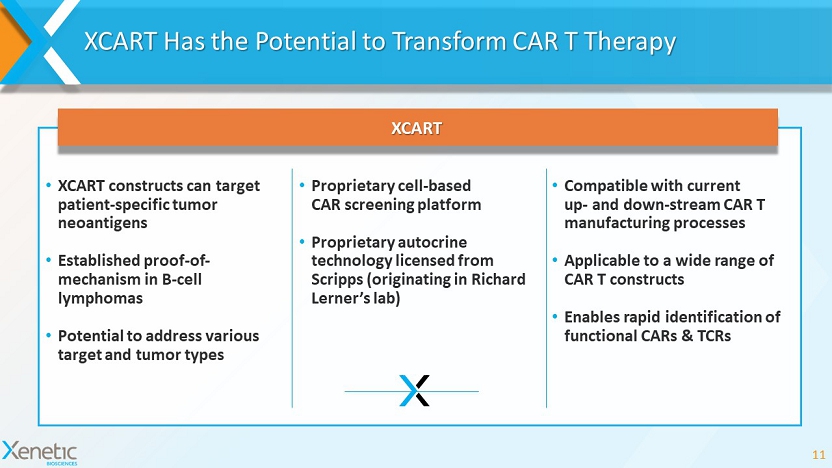
XCART Has the Potential to Transform CAR T Therapy 11 • XCART constructs can target patient - specific tumor neoantigens • Established proof - of - mechanism in B - cell lymphomas • Potential to address various target and tumor types • Compatible with current up - and down - stream CAR T manufacturing processes • Applicable to a wide range of CAR T constructs • Enables rapid identification of functional CARs & TCRs XCART • Proprietary cell - based CAR screening platform • Proprietary autocrine technology licensed from Scripps (originating in Richard Lerner’s lab)

XCART Platform Described in Science Advances 12
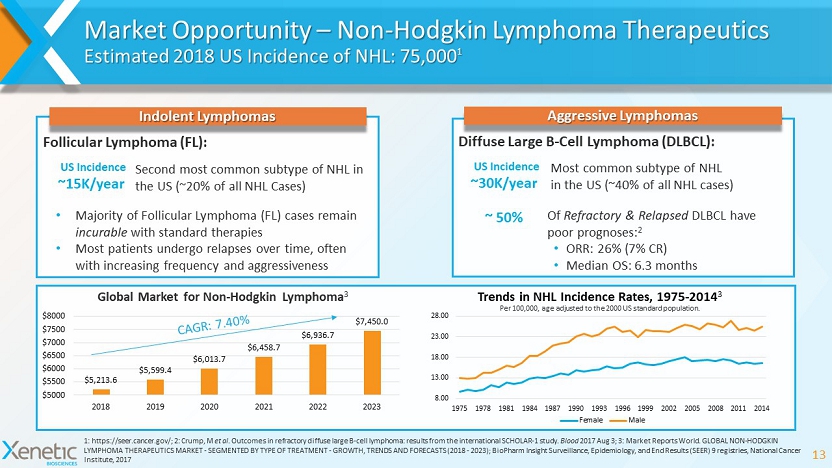
Market Opportunity – Non - Hodgkin Lymphoma Therapeutics Estimated 2018 US Incidence of NHL: 75,000 1 13 1: https://seer.cancer.gov/; 2: Crump, M et al . Outcomes in refractory diffuse large B - cell lymphoma: results from the international SCHOLAR - 1 study. Blood 2017 Aug 3; 3: Market Reports World. GLOBAL NON - HODGKIN LYMPHOMA THERAPEUTICS MARKET - SEGMENTED BY TYPE OF TREATMENT - GROWTH, TRENDS AND FORECASTS (2018 - 2023); BioPharm Insight Surveillance, Epidemiology, and End Results (SEER) 9 registries, National Cancer Institute, 2017 XBIO - 101 Aggressive Lymphomas Most common subtype of NHL in the US (~40% of all NHL cases) ~30K/year ~ 50% Diffuse Large B - Cell Lymphoma (DLBCL): Of Refractory & Relapsed DLBCL have poor prognoses: 2 • ORR: 26% (7% CR) • Median OS: 6.3 months Follicular Lymphoma (FL): Second most common subtype of NHL in the US (~20% of all NHL Cases) • Majority of Follicular Lymphoma (FL) cases remain incurable with standard therapies • Most patients undergo relapses over time, often with increasing frequency and aggressiveness $5,213.6 $5,599.4 $6,013.7 $6,458.7 $6,936.7 $7,450.0 $5000 $5500 $6000 $6500 $7000 $7500 $8000 2018 2019 2020 2021 2022 2023 Global Market for Non - Hodgkin Lymphoma 3 8.00 13.00 18.00 23.00 28.00 1975 1978 1981 1984 1987 1990 1993 1996 1999 2002 2005 2008 2011 2014 Trends in NHL Incidence Rates, 1975 - 2014 3 Female Male Per 100,000, age adjusted to the 2000 US standard population. Indolent Lymphomas US Incidence ~15K/year US Incidence
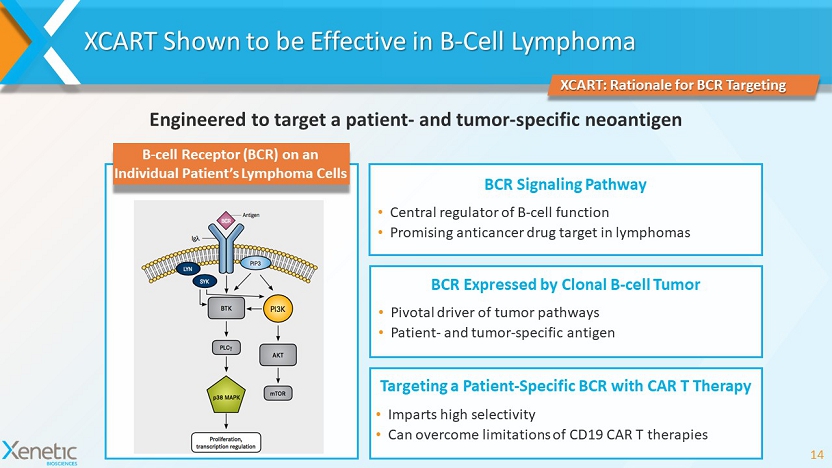
XCART Shown to be Effective in B - Cell Lymphoma 14 XCART: Rationale for BCR Targeting Engineered to target a patient - and tumor - specific neoantigen B - cell Receptor (BCR) on an Individual Patient’s Lymphoma Cells BCR Signaling Pathway • Central regulator of B - cell function • Promising anticancer drug target in lymphomas BCR Expressed by Clonal B - cell Tumor • Pivotal driver of tumor pathways • Patient - and tumor - specific antigen Targeting a Patient - Specific BCR with CAR T Therapy • Imparts high selectivity • Can overcome limitations of CD19 CAR T therapies
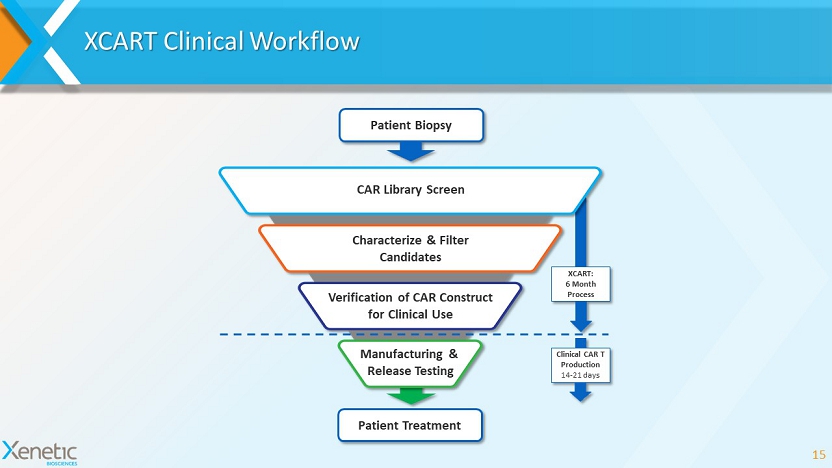
Clinical CAR T Production 14 - 21 days XCART: 6 Month Process Manufacturing & Release Testing CAR Library Screen Characterize & Filter Candidates Verification of CAR Construct for Clinical Use Patient Treatment Patient Biopsy XCART Clinical Workflow 15
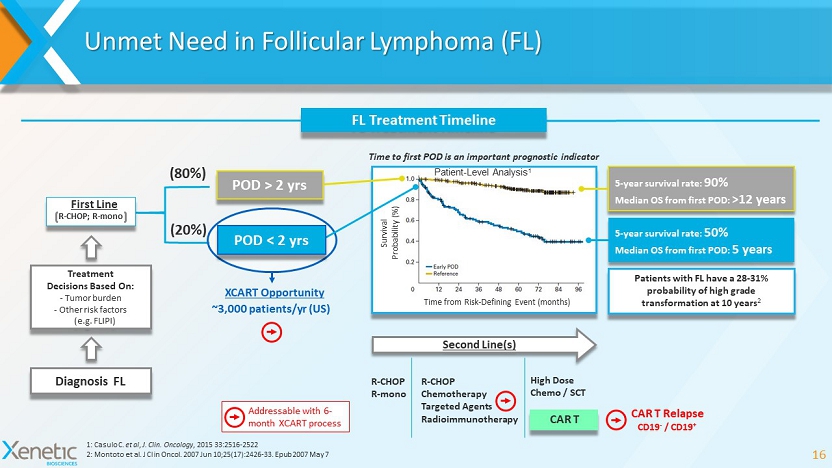
Addressable with 6 - month XCART process Unmet Need in Follicular Lymphoma (FL) 16 1: Casulo C. et al , J. Clin. Oncology , 2015 33:2516 - 2522 2: Montoto et al. J Clin Oncol. 2007 Jun 10;25(17):2426 - 33. Epub 2007 May 7 Time from Risk - Defining Event (months) Survival Probability (%) FL Treatment Timeline 5 - year survival rate: 90% Median OS from first POD: >12 years 5 - year survival rate: 50% Median OS from first POD: 5 years POD > 2 yrs (80%) (20%) R - CHOP; R - mono First Line Diagnosis FL Treatment Decisions Based On: - Tumor burden - Other risk factors (e.g. FLIPI) Time to first POD is an important prognostic indicator CAR T Second Line(s) R - CHOP R - mono R - CHOP Chemotherapy Targeted Agents Radioimmunotherapy High Dose Chemo / SCT Patients with FL have a 28 - 31% probability of high grade transformation at 10 years 2 POD < 2 yrs XCART Opportunity Patient - Level Analysis 1 ~3,000 patients/ yr (US) CAR T Relapse CD19 - / CD19 +
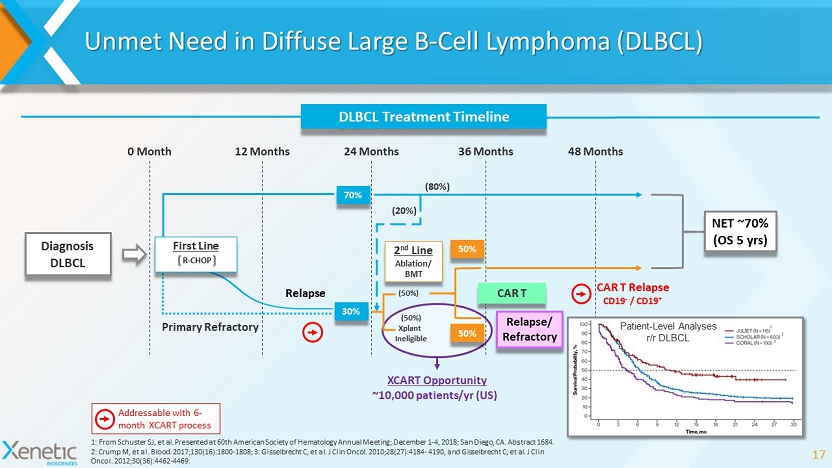
Unmet Need in Diffuse Large B - Cell Lymphoma (DLBCL) 17 12 Months 48 Months 24 Months 36 Months 0 Month (50%) Xplant Ineligible (50%) Relapse (20%) (80%) Diagnosis DLBCL 70% 2 nd Line Ablation/ BMT Relapse/ Refractory 50% 50% DLBCL Treatment Timeline 30% Primary Refractory XCART Opportunity 1: From Schuster SJ, et al. Presented at 60th American Society of Hematology Annual Meeting; December 1 - 4, 2018; San Diego, CA. Abstract 1684. 2: Crump M, et al. Blood. 2017;130(16):1800 - 1808; 3: Gisselbrecht C, et al. J Clin Oncol. 2010;28(27):4184 - 4190, and Gisselbrecht C, et al. J Clin Oncol. 2012;30(36):4462 - 4469. 1 2 3 R - CHOP First Line Patient - Level Analyses r/r DLBCL NET ~70% (OS 5 yrs ) ~10,000 patients/ yr (US) CAR T CAR T Relapse CD19 - / CD19 + Addressable with 6 - month XCART process
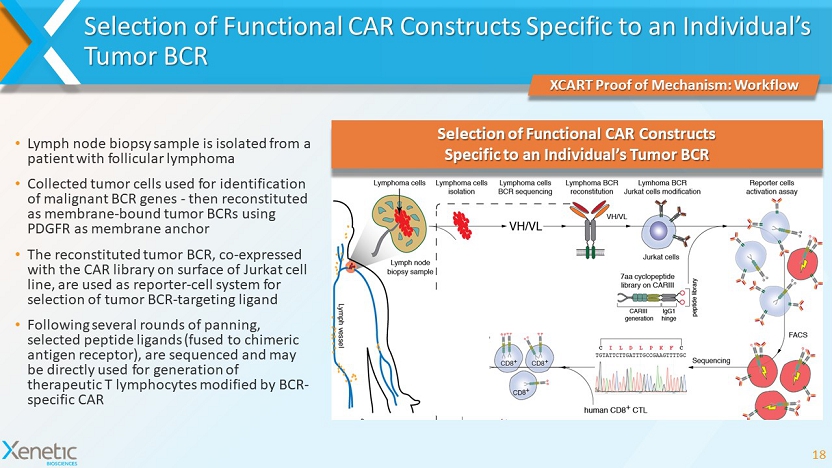
Selection of Functional CAR Constructs Specific to an Individual’s Tumor BCR 18 XCART Proof of Mechanism: Workflow Selection of Functional CAR Constructs Specific to an Individual’s Tumor BCR • Lymph node biopsy sample is isolated from a patient with follicular lymphoma • Collected tumor cells used for identification of malignant BCR genes - then reconstituted as membrane - bound tumor BCRs using PDGFR as membrane anchor • The reconstituted tumor BCR, co - expressed with the CAR library on surface of Jurkat cell line, are used as reporter - cell system for selection of tumor BCR - targeting ligand • Following several rounds of panning, selected peptide ligands (fused to chimeric antigen receptor), are sequenced and may be directly used for generation of therapeutic T lymphocytes modified by BCR - specific CAR
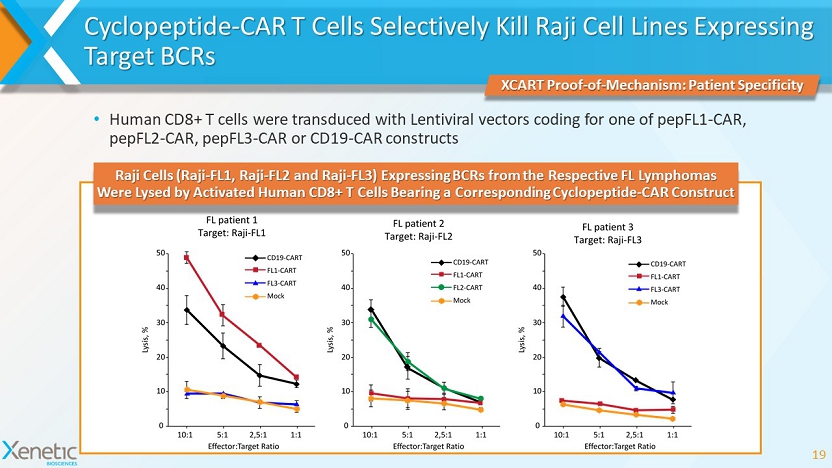
Cyclopeptide - CAR T Cells Selectively Kill Raji Cell Lines Expressing Target BCRs • Human CD8+ T cells were transduced with Lentiviral vectors coding for one of pepFL1 - CAR, pepFL2 - CAR, pepFL3 - CAR or CD19 - CAR constructs 19 XCART Proof - of - Mechanism: Patient Specificity Raji Cells (Raji - FL1, Raji - FL2 and Raji - FL3) Expressing BCRs from the Respective FL Lymphomas Were Lysed by Activated Human CD8+ T Cells Bearing a Corresponding Cyclopeptide - CAR Construct
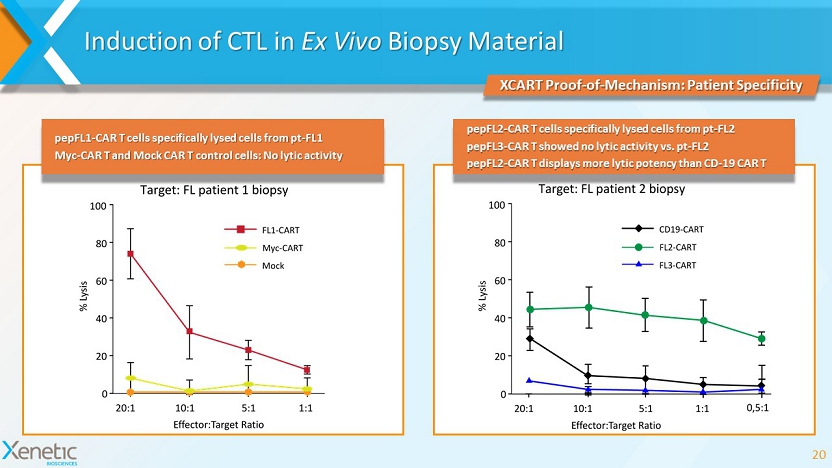
Induction of CTL in Ex Vivo Biopsy Material 20 XCART Proof - of - Mechanism: Patient Specificity pepFL1 - CAR T cells specifically lysed cells from pt - FL1 Myc - CAR T and Mock CAR T control cells: No lytic activity pepFL2 - CAR T cells specifically lysed cells from pt - FL2 pepFL3 - CAR T showed no lytic activity vs. pt - FL2 pepFL2 - CAR T displays more lytic potency than CD - 19 CAR T
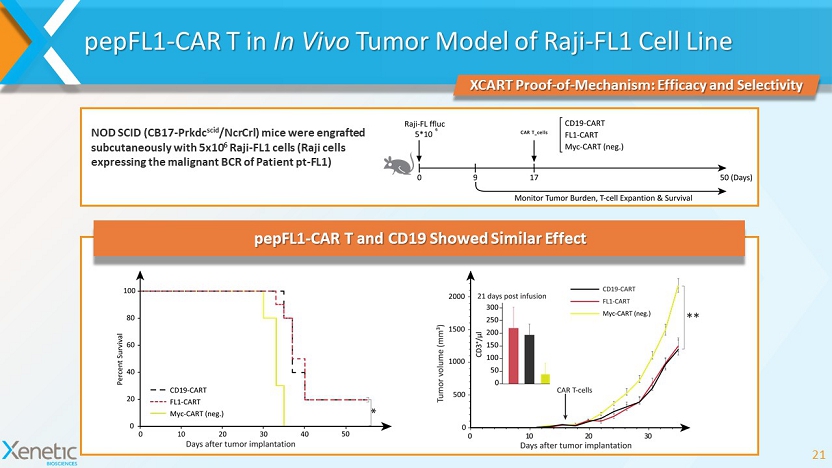
pepFL1 - CAR T in In Vivo Tumor Model of Raji - FL1 Cell Line 21 XCART Proof - of - Mechanism: Efficacy and Selectivity NOD SCID (CB17 - Prkdc scid / NcrCrl ) mice were engrafted subcutaneously with 5x10 6 Raji - FL1 cells (Raji cells expressing the malignant BCR of Patient pt - FL1) pepFL1 - CAR T and CD19 Showed Similar Effect
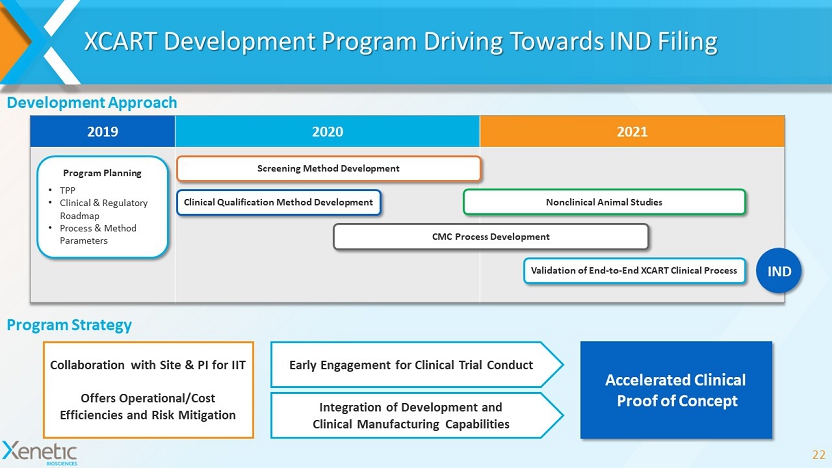
XCART Development Program Driving Towards IND Filing 22 2019 2020 2021 Screening Method Development Validation of End - to - End XCART Clinical Process Clinical Qualification Method Development Nonclinical Animal Studies CMC Process Development Development Approach Program Planning • TPP • Clinical & Regulatory Roadmap • Process & Method Parameters IND Program Strategy Collaboration with Site & PI for IIT Offers Operational/Cost Efficiencies and Risk Mitigation Accelerated Clinical Proof of Concept Early Engagement for Clinical Trial Conduct Integration of Development and Clinical Manufacturing Capabilities
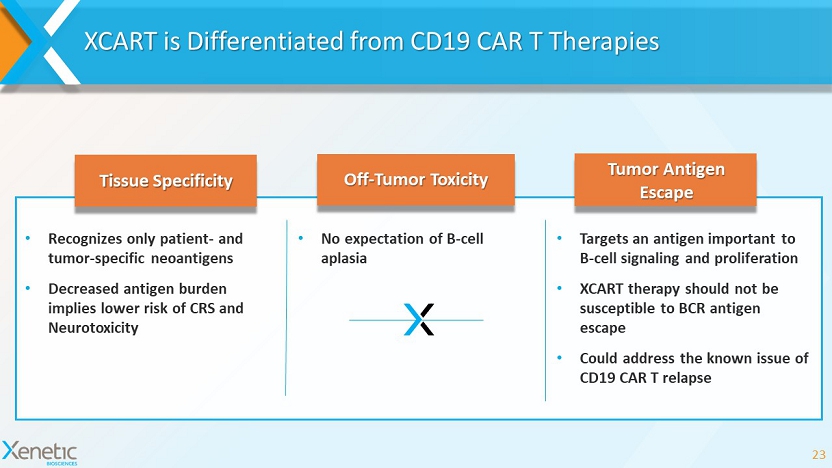
XCART is Differentiated from CD19 CAR T Therapies 23 Tissue Specificity Off - Tumor Toxicity Tumor Antigen Escape • Recognizes only patient - and tumor - specific neoantigens • Decreased antigen burden implies lower risk of CRS and Neurotoxicity • No expectation of B - cell aplasia • Targets an antigen important to B - cell signaling and proliferation • XCART therapy should not be susceptible to BCR antigen escape • Could address the known issue of CD19 CAR T relapse

PolyXen ™ PSA Technology Platform Enables Next Generation Biologic Drugs
PolyXen : Next Generation Delivery Platform for Biologics 25 Polysialylation employs the biological polymer polysialic acid (PSA) to modulate the pharmacokinetic and pharmacodynamic profiles of protein drugs Seeking to build a pipeline of partnerships utilizing PolyXen Key Features • Retention of native protein conformation • Non - immunogenic • Biodegradable • Fewer injections • Improved protease stability • Improved thermal stability • Broad patent cover License Agreement • Exclusive License Agreement with Takeda in the field of coagulation disorders • Granted right to Takeda to grant a nonexclusive sublicense to certain patents related to PolyXen • Received $7.5 million upfront payment • Single digit royalties based on net sales • Royalty stream could commence by end of 2019 • One active development program Broad Utility • Clinically demonstrated to extend half - life of therapeutic proteins • Applicable to franchise extensions as well as candidates in development • Potential utility in other molecule classes such as peptides and small molecules
Summary 26 1: Acquisition, subject to the satisfaction of the closing conditions, is expected to close in the first half of 2019. Please se e our Registration Statement on Form S - 4 for more details • Innovative XCART platform 1 addressing high - value oncology market • PolyXen platform: next generation delivery for biologics PolyXen Takeda Agreement • Takeda granted a nonexclusive sublicense to certain patents • Royalty stream could commence by end of 2019 • Active program with Takeda and potential for additional partnerships XCART Platform Lead Program Targeting $5 Billion Initial Opportunity in B - Cell Non - Hodgkin Lymphoma • Proof - of - mechanism and preclinical evidence of target specificity

Enhancing lives with transformative therapies www.xeneticbio.com NASDAQ: XBIO Supplemental Information
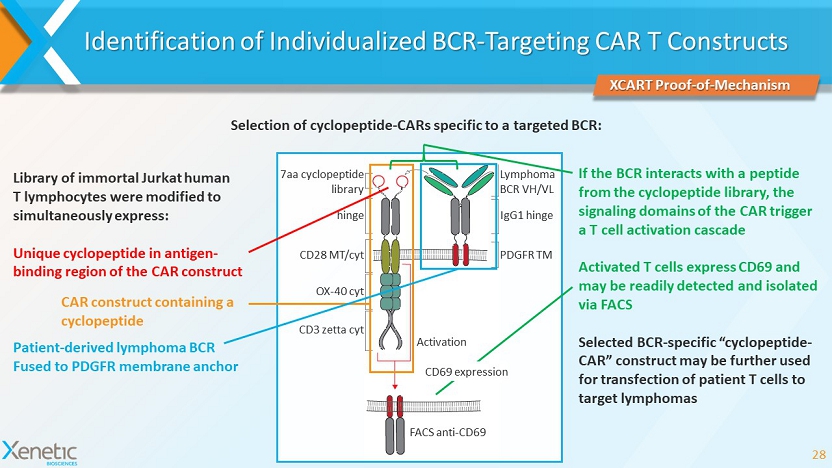
Identification of Individualized BCR - Targeting CAR T Constructs 28 Selection of cyclopeptide - CARs specific to a targeted BCR: 7aa cyclopeptide library hinge CD28 MT/ cyt OX - 40 cyt CD3 zetta cyt Lymphoma BCR VH/VL IgG1 hinge PDGFR TM Activation FACS anti - CD69 Library of immortal Jurkat human T lymphocytes were modified to simultaneously express: Unique cyclopeptide in antigen - binding region of the CAR construct If the BCR interacts with a peptide from the cyclopeptide library, the signaling domains of the CAR trigger a T cell activation cascade Activated T cells express CD69 and may be readily detected and isolated via FACS Selected BCR - specific “cyclopeptide - CAR” construct may be further used for transfection of patient T cells to target lymphomas XCART Proof - of - Mechanism CD69 expression CAR construct containing a cyclopeptide Patient - derived lymphoma BCR Fused to PDGFR membrane anchor
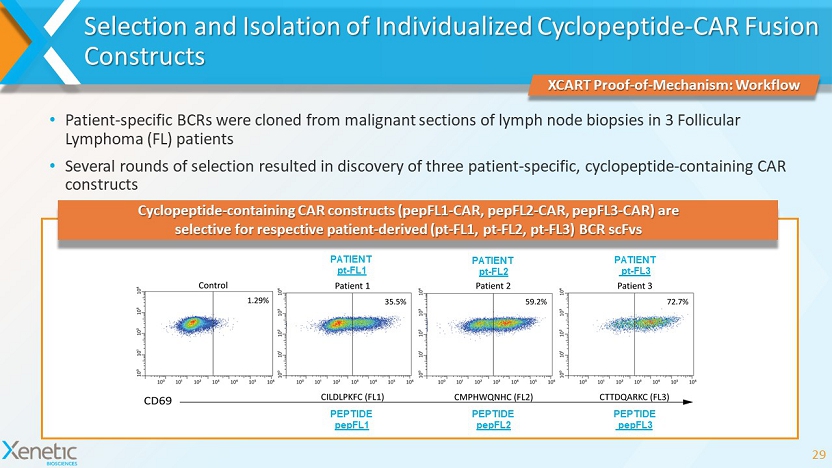
• Patient - specific BCRs were cloned from malignant sections of lymph node biopsies in 3 Follicular Lymphoma (FL) patients • Several rounds of selection resulted in discovery of three patient - specific, cyclopeptide - containing CAR constructs Selection and Isolation of Individualized Cyclopeptide - CAR Fusion Constructs 29 XCART Proof - of - Mechanism: Workflow Cyclopeptide - containing CAR constructs (pepFL1 - CAR, pepFL2 - CAR, pepFL3 - CAR) are selective for respective patient - derived (pt - FL1, pt - FL2, pt - FL3) BCR scFvs PATIENT pt - FL1 PATIENT pt - FL2 PATIENT pt - FL3 PEPTIDE pepFL1 PEPTIDE pepFL2 PEPTIDE pepFL3
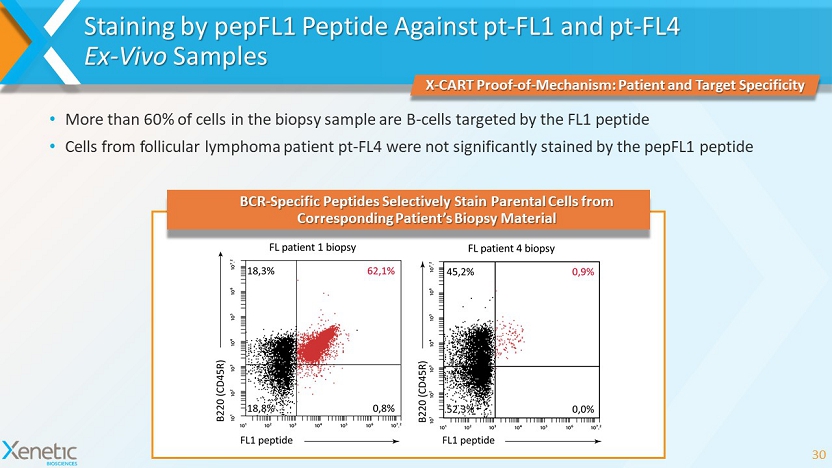
Staining by pepFL1 Peptide Against pt - FL1 and pt - FL4 Ex - Vivo Samples 30 X - CART Proof - of - Mechanism: Patient and Target Specificity BCR - Specific Peptides Selectively Stain Parental Cells from Corresponding Patient’s Biopsy Material • More than 60% of cells in the biopsy sample are B - cells targeted by the FL1 peptide • Cells from follicular lymphoma patient pt - FL4 were not significantly stained by the pepFL1 peptide
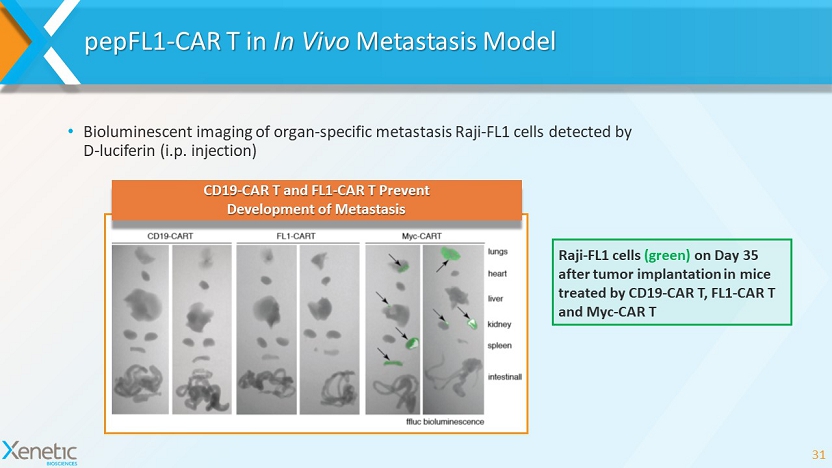
pepFL1 - CAR T in In Vivo Metastasis Model 31 Raji - FL1 cells (green) on Day 35 after tumor implantation in mice treated by CD19 - CAR T, FL1 - CAR T and Myc - CAR T CD19 - CAR T and FL1 - CAR T Prevent Development of Metastasis • Bioluminescent imaging of organ - specific metastasis Raji - FL1 cells detected by D - luciferin ( i.p. injection)
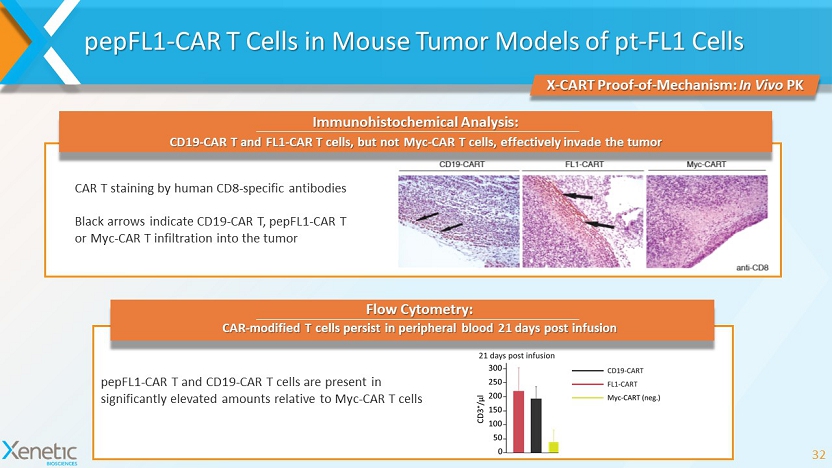
pepFL1 - CAR T Cells in Mouse Tumor Models of pt - FL1 Cells 32 X - CART Proof - of - Mechanism: In Vivo PK CAR T staining by human CD8 - specific antibodies Black arrows indicate CD19 - CAR T, pepFL1 - CAR T or Myc - CAR T infiltration into the tumor pepFL1 - CAR T and CD19 - CAR T cells are present in significantly elevated amounts relative to Myc - CAR T cells Immunohistochemical Analysis: CD19 - CAR T and FL1 - CAR T cells, but not Myc - CAR T cells, effectively invade the tumor CAR - modified T cells persist in peripheral blood 21 days post infusion Flow Cytometry: