
As filed with the Securities and Exchange Commission on October 21, 2016
Registration No. 333-211249
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_________________________
AMENDMENT NO. 6
TO
FORM S-1
REGISTRATION STATEMENT
Under
The Securities Act of 1933
_________________________
XENETIC BIOSCIENCES, INC.
(Exact name of registrant as specified in its charter)
_________________________
| Nevada | 2834 | 45-2952962 |
|
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
99 Hayden Ave, Suite 230
Lexington, Massachusetts 02421
(781) 778-7720
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
_________________________
M. Scott Maguire
President and Chief Executive Officer
Xenetic Biosciences, Inc.
99 Hayden Ave, Suite 230
Lexington, Massachusetts 02421
(781) 778-7720
(Name, address, including zip code, and telephone number, including area code, of agent for service of process)
_________________________
Copies to:
|
Mitchell D. Goldsmith |
Michael D. Maline New York Times Building 620 Eighth Avenue New York, New York 10036 (212) 813-8800 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, check the following box. [X]
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [_]
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [_]
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [_]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | [_] | Accelerated filer | [_] |
| Non-accelerated filer (Do not check if a smaller reporting company) [_] | Smaller reporting company | [X] | |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
| ||
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED OCTOBER 21, 2016 |
|
500,000 units consisting of One Share of Convertible Series B Preferred Stock and a Class A Warrant to Purchase One Share of Common Stock and 2,000,000 units consisting of One Share of Convertible Series B Preferred Stock and a Class B Warrant to Purchase One Share of Common Stock | ||

We are offering up to 2,500,000 units, 500,000 of which units consist of one share of convertible Series B Preferred Stock and a Class A Warrant to purchase one share of common stock with an exercise price of $_____ per warrant, and 2,000,000 of which units consist of one share of convertible Series B Preferred Stock and a Class B Warrant to purchase one share of common stock, each with an exercise price of $_____ per warrant. The Class A Warrants will be exerciseable six months after issuance and will expire on the fifth anniversary of the original issuance date. The Class B Warrants will be exerciseable immediately upon issuance and will expire on the fifth anniversary of the original issuance date. Each unit will be sold at a negotiated price of $___ per unit. The units will not be issued or certificated. The shares of preferred stock and the warrants are immediately separable and will be issued separately, but will be purchased together in this offering. The shares of our common stock issuable from time to time upon conversion of the Series B Preferred Stock and exercise of the warrants are also being offered pursuant to this prospectus. The Class A Warrants will be issued to existing institutional investors of our common stock. All other investors will receive Class B Warrants.
Our common stock is quoted on the OTCQB marketplace under the symbol “XBIO.” We have applied to list our common stock on the NASDAQ Capital Market under the symbol “XBIO.” The closing of this offering is dependent upon the approval for the listing of our common stock on the NASDAQ Capital Market, although this offering will close before such listing. No assurance can be given that our application will be approved. There is no established public trading market for our common stock and the share prices on an over-the-counter marketplace may not be indicative of the market price of our common stock on the NASDAQ Capital Market. On October 20, 2016, the last reported sale price of our common stock on the OTCQB was $4.40 per share. In addition, we do not intend to apply for listing of the Series B Preferred Stock or the Class A Warrants and Class B Warrants on any national securities exchange or other trading market. Without an active trading market, the liquidity of the Series B Preferred Stock and warrants will be limited.
We are an emerging growth company as that term is used in the Jumpstart Our Business Startups Act of 2012 and, as such, have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings. See “Prospectus Summary—Implications of Being an Emerging Growth Company.”
One of our existing institutional investors has a contractual obligation to purchase an aggregate of up to approximately $6,000,000 in units in this offering at the initial public offering price and on the same terms as the other purchasers in this offering.
Investing in the offered securities involves a high degree of risk. See “Risk Factors” of this prospectus for a discussion of information that you should consider before investing in our securities.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
| Per Unit | Total | ||
| Public Offering Price | $ | $ | |
| Underwriting Discount(1) | $ | $ | |
| Proceeds, Before Expenses, to Us | $ | $ | |
| (1) | See “Underwriting” for a description of compensation payable to the underwriter. | ||
The underwriter expects to deliver the units to purchasers in the offering on or about , 2016.
LADENBURG THALMANN
The Date of This Prospectus is: , 2016
TABLE OF CONTENTS
| Prospectus Summary | 1 |
| The Offering | 10 |
| Risk factors | 14 |
| Special Note Regarding Forward-Looking Statements | 44 |
| Use of proceeds | 45 |
| Dividend policy | 46 |
| Market Price of Our Common Stock | 47 |
| Capitalization | 49 |
| Dilution | 50 |
| Selected CONSOLIDATED Financial Data | 51 |
| Management’s Discussion and Analysis of Financial Condition and Results of Operations | 53 |
| Business | 69 |
| Management | 100 |
| Executive Compensation | 105 |
| Certain Relationships and Related Party Transactions | 109 |
| Principal Stockholders | 112 |
| Description of Capital Stock | 114 |
| Certain material U.S. Federal Income Tax Considerations | 121 |
| Underwriting | 128 |
| Legal Matters | 130 |
| Experts | 130 |
| CHANGES IN REGISTRANT’S CERTIFYING PUBLIC ACCOUNTANT | 130 |
| Where You Can Find More Information | 131 |
| INCORPORATION OF DOCUMENTS BY REFERENCE | 131 |
| Index to Financial Statements | F-1 |
_____________________________
We have not authorized anyone to provide you with any information or to make any representation, other than those contained in this prospectus or any free writing prospectus we have prepared. We take no responsibility for, and provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the shares offered hereby, but only in jurisdictions where it is lawful to so do. The information contained in this prospectus is accurate only as of its date, regardless of the time of delivery of this prospectus or of any sale of our common stock.
It is important for you to read and consider all information contained in this prospectus in making your investment decision. You should also read and consider the information in the documents to which we have referred you to in the sections entitled “Where You Can Find More Information” in this prospectus.
Neither we nor the underwriter have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than the United States. You are required to inform yourself about, and to observe any restrictions relating to, this offering and the distribution of this prospectus.
Our logo and some of our trademarks and tradenames are used in this prospectus. This prospectus also includes trademarks, tradenames and service marks that are the property of others. Solely for convenience, trademarks, tradenames and service marks referred to in this prospectus may appear without the ®, ™ and SM symbols. References to our trademarks, tradenames and service marks are not intended to indicate in any way that we will not assert to the fullest extent under applicable law our rights or the rights of the applicable licensor, nor that respective owners to other intellectual property rights will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by any other companies. Since our patents are either held by us or our wholly-owned subsidiaries, we will not distinguish between patents held by us or our subsidiaries in this prospectus.
The market data and certain other statistical information used throughout this prospectus are based on independent industry publications, reports by market research firms or other independent sources that we believe to be reliable sources. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We are responsible for all of the disclosure contained in this prospectus, and we believe these industry publications and third-party research, surveys and studies are reliable. While we are not aware of any misstatements regarding any third-party information presented in this prospectus, their estimates, in particular, as they relate to projections, involve numerous assumptions, are subject to risks and uncertainties, and are subject to change based on various factors, including those discussed under the section entitled “Risk Factors” and elsewhere in this prospectus. Some data are also based on our good faith estimates.
______________________
| i |
Prospectus Summary
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before investing in our common stock, you should carefully read this entire prospectus, including our financial statements and the related notes thereto. You should also consider, among other things, the matters described under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” in each case appearing elsewhere in this prospectus.
Unless otherwise stated, all references to “us,” “our,” “Xenetic,” “we,” the “Company” and similar designations refer to Xenetic Biosciences, Inc. and its subsidiaries, as well as Xenetic U.K.
Overview
We are a clinical-stage biopharmaceutical company focused on discovery, research and development of next-generation biologic drugs and novel orphan oncology therapeutics that may contribute to improvements in global human health. Our 200+ patent portfolio covers next generation biologic drugs and novel oncology therapeutics and provides protection for our current drug candidates and positions as well as strategic partnership and commercialization opportunities.
Our objective is to leverage our portfolio to maximize out-license opportunities that generate working capital to both build incremental shareholder value and provide funding necessary to clinically develop our orphan oncology drug candidate pipeline through to market launch.
Our lead product candidates include ErepoXen, a polysialylated form of erythropoietin (EPO) for the treatment of anemia in pre-dialysis patients with chronic kidney disease, and U.S. Food and Drug Administration (FDA) orphan designated oncology therapeutics Virexxa and OncoHist for the treatment of progesterone receptor negative endometrial cancer and refractory acute myeloid leukemia, respectively.
We are also working together with Shire plc (Shire, NASDAQ:SHPG), formerly Baxalta and before that Baxter Healthcare, to develop a novel series of polysialylated blood coagulation factors, including a next generation BAX 826. This collaboration relies on Xenetic's PolyXen technology to conjugate PSA to therapeutic blood-clotting factors, with the goal of improving the pharmacokinetic profile and extending the active life of these biologic molecules. Shire is one of our largest shareholders having invested $10 million in our common stock during 2014. The agreement is an exclusive research, development and license agreement which grants Shire a worldwide, exclusive, royalty-bearing license to our PSA patented and proprietary technology in combination with Shire’s proprietary molecules designed for the treatment of blood and bleeding disorders. Under the agreement, we may receive regulatory and sales target payments for total potential milestone receipts of up to $100 million, plus royalties on sales.
Our Technology and Product Candidates
The Technologies
We incorporate our patented and proprietary technologies into a number of drug candidates currently under development either in-house or with biotechnology and pharmaceutical collaborators in order to create what we believe will be the next-generation of biologic drugs and therapeutics. While we primarily focus on researching and developing orphan oncology drugs, we also have significant economic and ownership interests in drugs being developed by our collaborators to treat, among others, hemophilia and anemia. Our patent portfolio spans four core proprietary technologies including two platforms, small molecules and biologics. The figure below depicts our current intellectual property, technologies and drug candidates.
| 1 |
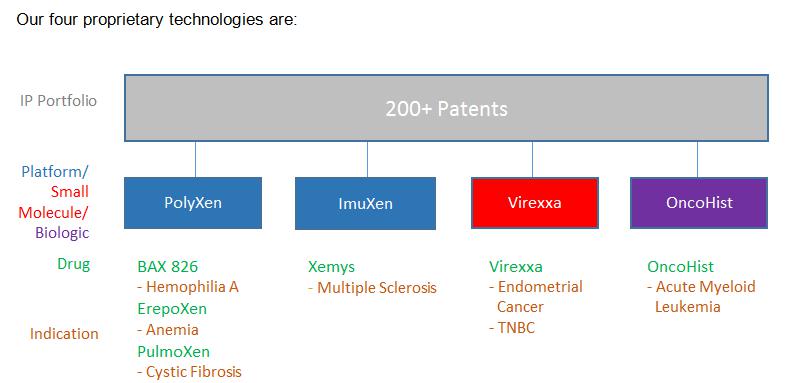
Our four proprietary technologies are:
| PolyXen | An enabling biological platform technology designed to extend the circulation in the human body for a variety of existing drug molecules and, thereby, to create potentially superior next generation drug candidates. PolyXen is based on the concept of polysialylation and utilizes polysialic acid, or PSA, which is a biopolymer, comprising a chain of sialic acid molecules. PSA is a natural constituent of the human body, though we obtain our PSA from a bacterial source. |
| Virexxa | A small molecule therapeutic with the potential to confer sensitivity to cancer cells to hormone therapeutics that are otherwise insensitive to such treatments. Virexxa, sodium cridanimod, belongs to a class of low-molecular weight synthetic interferon inducers. In addition to its immunomodulatory properties, Virexxa has been shown to increase levels of progesterone receptor expression in tumor tissue of patients who are progesterone receptor deficient, and thus may restore sensitivity of non-responsive endometrial cancers to hormonal (e.g., progestin) therapy. Based on preclinical observations, Virexxa may also be therapeutically relevant in other hormone-resistant cancers, such as triple-negative breast cancer. Virexxa has been granted an Orphan Drug Designation by the FDA, for treatment of progesterone receptor negative endometrial cancer in conjunction with progesterone therapy. |
| OncoHist | A novel therapeutic platform technology that utilizes the properties of modified human histone H1.3 for targeted cell necrosis or apoptosis programed cell death, which may enable OncoHist to treat a broad range of cancer indications. OncoHist, unlike many competing oncology therapies, is based on a molecule occurring naturally in the human body, in the cell nucleus, and is therefore expected to be less toxic and immunogenic than other oncology therapies. |
| ImuXen | A novel liposomal co-entrapment encapsulation technology designed to maximize both cell and immune system mediated responses. The technology is based on the co-entrapment of the nominated antigen(s) in a liposomal vesicle. The technology when applied may create new vaccines and improve the use and efficacy of certain existing human vaccines. |
These proprietary technologies may address unmet needs, improve the performance of existing drugs, and create new patentable drug candidates. All of our drug candidates are in the development stage and none have yet received regulatory approval for marketing in the U.S. by the FDA or by any applicable agencies in other countries.
| 2 |
In-House Research, Outside Services and Collaborations
We are focused on developing our pipeline of next generation bio-therapeutics and novel orphan oncology drugs based on our PolyXen, OncoHist and Virexxa proprietary technologies. In order to do this while efficiently managing our overhead, we rely on in-house research, services of contract manufacturers and contract research organizations and strategic collaborations. As such, continuous pipeline growth and advancement of technologies and drug candidates is dependent, in part, on several important collaborations and strategic arrangements with, among others:
| · | Shire, a global biopharmaceutical leader and significant shareholder of ours; | |
| · | SynBio LLC (SynBio), a Russian pharmaceutical company and significant shareholder of ours; | |
| · | PJSC Pharmsynthez (Pharmsynthez), a Russian pharmaceutical company and significant shareholder of ours; and | |
| · | Serum Institute of India Limited (Serum Institute), one of the world’s largest vaccine manufacturers and India’s largest biotech companies and significant shareholder of ours. |
Accordingly, in addition to our pipeline of next generation bio-therapeutics and novel orphan oncology drugs, we also have significant interests in drugs being developed by our collaborators to treat, among others, hemophilia and anemia, where we expect to collect milestone royalties. For further detail, please read the section entitled “Significant Co-Development Collaborations and Strategic Arrangements” on page 83.
Our Product Pipeline
Our product pipeline contains a number of our drug candidates under development in-house and drug candidates under development with biotechnology and pharmaceutical collaborators. The following chart summarizes key information regarding the current pharmaceutical products, organized by our corporate programs and our collaborators’ programs:
| Xenetic Corporate Programs | |||||||||
| Drug Candidate | Technology | Indication | ICH | Orphan Disease | Description, Status and Next Catalyst | Pre-clinical | Phase 1 | Phase 2 | Phase 3 |
| Virexxa | Virexxa | Endometrial Cancer | ✔ | ✔ | VIR-EC-01: Phase II trial in progress under a US IND (FDA Orphan Designation); Q4 2016 – expect US trial expanded protocol to commence | ||||
| ErepoXen | PolyXen | Anemia | ✔ | PSA-EPO-06: Phase II trial being conducted in Australia, South Africa and New Zealand. Cohort III in progress; Q4 2016 – expect report Phase 2 cohort 3 | |||||
| OncoHist | OncoHist | Acute Myeloid Leukemia | ✔ | ONC-AML-01: Pre-clinical studies and pre-IND meeting with the FDA is complete. Negotiations with contract manufacture and clinical research organizations are in progress (FDA Orphan Designation); H2 2017 – expect to file IND | |||||
| Virexxa | Virexxa | Triple Negative Breast Cancer | ✔ | VRX-BC-01: Pre-clinical studies under development; H2 2016 - expect to file IND | |||||
| Indicates programs completed. | ||
| Indicates programs in progress in such phase |
| Collaborative Partner Programs (alphabetical by clinical developer) | ||||||||||
| Drug Candidate | Technology | Sponsor | Indication | ICH | Orphan Disease | Description and Status | Pre-clinical | Phase 1 | Phase 2 | Phase 3 |
| BAX 826 | PolyXen | Shire | Hemophilia | ✔ | ✔ | PSA-FVIII: CTA in the U.K. for a Phase I/II clinical trial was approved. Clinical trial in the U.K. commenced in Q1 2016. | ||||
| PulmoXen | PolyXen | Pharmsynthez | Cystic Fibrosis | ✔ | PMO-CF-01: Phase I completed in Russia. A Phase II clinical trial is expected to start Q4 2016 in Russia. | |||||
| Xemys | ImuXen | Pharmsynthez | Multiple Sclerosis | IMU-MS-01: Phase I dose ranging study in Russia is complete. | ||||||
| Oxyntolong | PolyXen | Pharmsynthez | Type II Diabetes and Obesity | OXN: Phase 1 completed in Russia. Russian Phase 2 in progress with dose ranging studies completed. | ||||||
| ErepoXen | PolyXen | Serum Institute | Anemia | ✔ | PSA-EPO-03: Phase II(a) intravenous and subcutaneous human clinical trials conducted in India are complete. The study report is expected in Q2 2016. | |||||
| ErepoXen | PolyXen | SynBio | Anemia | PSA-EPO-05: Russian Phase II(b)/III in progress. | ||||||
| OncoHist | OncoHist | SynBio | AML | ✔ | Onc-AML-02: Russian Phase II is on hold pending protocol revision due to a change in Russian Standard of Care requirements. | |||||
| OncoHist | OncoHist | SynBio | NHL | Onc-NHL-01: Russian Phase II dose ranging studies are completed in Russia. | ||||||
| Indicates programs completed. | ||
| Indicates programs in progress in such phase |
| 3 |
BAX 826
Pursuant to our exclusive license agreement with Shire, one of our largest shareholders, Shire will develop a novel series of polysialylated blood coagulation factors, including BAX 826. BAX 826 relies on our PolyXen platform technology to develop its next generation, extended half-life treatment based on ADVATE (rFVIII), the world’s most prescribed treatment for blood coagulation disorders. This product candidate has the goal of improving bleed protection in Hemophilia A patients while potentially offering once weekly or even less frequent infusions. Current market-approved, next generation treatments require at least twice-weekly administration. Shire has commenced dosing in a Phase I clinical trial for BAX 826 in the U.K.
Virexxa
On November 13, 2015, we entered into an Asset Purchase Agreement, or the Kevelt APA, with AS Kevelt, an Estonian biotech company, or Kevelt, and Pharmsynthez. Pursuant to the Kevelt APA, Kevelt and Pharmsynthez, transferred to us certain intellectual property rights with respect to Virexxa, and the worldwide rights to develop, market and license Virexxa for certain uses, except for excluded uses within the Commonwealth of Independent States (CIS) in exchange for, among others, 3,378,788 shares of our common stock. Virexxa is a Phase II oncology drug candidate which is under investigation for the treatment of certain endometrial cancers. As part of this total consideration, we also acquired Kevelt's U.S. Orphan Drug designation for the use of Virexxa in the treatment of progesterone receptor negative endometrial cancer in conjunction with progesterone therapy.
Virexxa is our most advanced candidate with an orphan designation for a subset of endometrial cancers and an Investigational New Drug, or IND, in effect for Phase II clinical trials in the U.S. and certain territories in Eastern Europe. While Virexxa (a sodium cridanimod), belongs to a class of low-molecular weight synthetic interferon, or IFN, inducers and is primarily used in a wide range of therapeutic areas such as antiviral, antibacterial, antitumor, and inflammatory indications due to its ability to modify or regulate one or more immune system functions, Virexxa may prove to be therapeutically relevant in hormone-resistant cancers by increasing the levels of progesterone receptor, or PR, expression in tumor tissue of patients who are PR deficient. As such, it may restore the sensitivity of non-responsive endometrial cancers to hormonal (e.g., progestin) therapy. Accordingly, we are pursuing the use of Virexxa for endometrial cancer.
Our decision to investigate Virexxa for the treatment of endometrial cancer was based in part on the history of sodium cridanimod in preclinical and clinical research conducted by others, including 22 clinical trials conducted and completed in Russia by other clinical developers that assessed the efficacy and safety of sodium cridanimod. Sodium cridanimod has been authorized and marketed in the Russian Federation for 20 years and 11 million doses have been sold for non-cancer indications. Sodium cridanimod has been authorized for medicinal use in the Russian Federation for 21 years and over one million packages have been sold for non-cancer indications. Virexxa is also known under the brand names Neovir, Camedon and Primavir.
Virexxa has been granted an Orphan Drug Designation by the FDA for treatment of progesterone receptor negative endometrial cancer in conjunction with progesterone therapy. Because of the extensive clinical testing and market history of sodium cridanimod, we were able to proceed directly to a Phase II study under our IND for the efficacy of use of sodium cridanimod in conjunction with progestin therapy in a population of patients with recurrent or persistent PR-negative endometrial cancer. This study is currently active under the same IND in Belarus and Ukraine.
| 4 |
ErepoXen
Our drug candidate that is the second most advanced in our clinical pipeline is ErepoXen polysialylated erythropoietin (PSA-EPO). ErepoXen uses our PolyXen platform technology for the treatment of anemia in chronic kidney disease, or CKD, patients. It is designed to reduce the dosing frequency by extending circulation time of the therapeutic in the body. It is potentially a drug with a substantial global market.
In addition to researching and developing ErepoXen ourselves, SynBio and Serum Institute have collaborative arrangements to develop and launch ErepoXen in limited markets pursuant to which we will collect royalties.
Serum Institute conducted Phase I and Phase II clinical trials in 95 human subjects. These safety trials, which had no significant adverse events, provided Xenetic with the data to commence a Phase II, repeat dosing, ICH compliant clinical trial for ErepoXen in Australia, New Zealand and South Africa for chronic kidney disease patients not on dialysis. We anticipate conducting three cohorts, of which we have completed two cohorts and fully recruited the third cohort. Each cohort represents an increased dose of ErepoXen that is given on a repeat schedule until therapeutic levels of hemoglobin are achieved. Both completed cohorts had no drug-related significant adverse events. In order to accelerate and finalize the Phase II clinical trial, we opened a South African arm for the third cohort.
Our collaborator, the Serum Institute, finished Phase I/II clinical trials in India of ErepoXen for in-center-dialysis patients. Serum Institute indicated that it will use its data from the Phase I/II clinical trials and data generated from Xenetic’s Phase II trial to further progress clinical trials of ErepoXen in India. In addition, SynBio applied for and received regulatory approval for ErepoXen Phase II(b)/III human clinical trials in Russia, which are in progress. SynBio has indicated that it will commence commercialization and marketing stage of ErepoXen in the Russian and CIS markets subject to approval in such markets.
OncoHist for AML
The next most advanced drug candidate is OncoHist, which utilizes the properties of modified human histone H1.3 for targeted cell killing. OncoHist for AML is for the treatment of relapsed or resistant acute myeloid leukemia (AML). We are currently researching and developing OncoHist for AML and we anticipate filing an IND application for OncoHist for AML subject to funding.
We have a sponsored research agreement with Dana Farber Cancer Institute intended to elucidate OncoHist’s mechanism of action as well as to characterize the responsiveness of various AML cell lines to OncoHist. Dr. Richard Stone, MD, Professor of Medicine at Harvard Medical School and Clinical Director of the Adult Leukemia Program at Dana-Farber Cancer Institute, presented data at the 2014 American Society of Hematology meeting (Blood, 2014 124(21):3604 OncoHist, an rh Histone 1.3, Is Cytotoxic to Acute Myeloid Leukemia Cells and Results in Altered Downstream Signaling).
We completed non-clinical toxicity studies of OncoHist guided, in part, by clinical data supplied by SynBio and SymbioTec, GmbH, a German company acquired by Xenetic in 2012. In August 2015, we had a productive, in-person pre-IND meeting with the FDA where manufacturing and clinical matters were addressed including guidance from the FDA regarding inclusion of an additional indication besides AML in our planned Phase I clinical trial.
Pipeline Expansion Opportunities
Operating under Xenetic licenses within their home markets, our collaborators generate pre-clinical and clinical data related to our technologies across a wide spectrum of therapeutic areas. Under these agreements, we retain all rights for major markets and co-owns the clinical data. We, therefore, have the opportunity to utilize the data in our decision making process regarding commercialization in major markets. For example, we expect to be able to utilize the results from substantially all of our clinical toxicity data and other clinical data generated in the development of OncoHist, Virexxa, ImuXen and PolyXen for a variety of orphan oncology indications and next generation biologic drugs.
For example, we believe we may be able to develop Virexxa for triple-negative breast cancer (TNBC) indications. Results from preclinical and exploratory studies conducted by a collaborative partner suggest that Virexxa can up-regulate (i.e., increase the levels of) estrogen receptor (ER) in certain tissue types. Proof of concept studies are being planned to investigate additional therapeutic opportunities for Virexxa in other hormone-resistant tumor types than endometrial cancer, including TNBCs.
| 5 |
We also believe that the nature of our technologies, including the PolyXen and ImuXen platforms, will allow us to pursue additional drug candidates for new indications based on existing and future scientific data.
Our Strategy
Our goal is to become a leader in the development of novel orphan oncology drugs while leveraging our proprietary delivery technology as a vehicle for creating next generation bio-therapeutics.
Our strategy is to pursue a continuous and ongoing effort of out-licensing our PolyXen platform technology to drive short-term, incremental shareholder value and generate working capital to assist in providing the funding required to support our long-term development of orphan oncology drug candidates through regulatory approval and commercialization.
We advance our PolyXen platform technology through collaborative out-license arrangements with global pharmaceutical companies that can apply the resources necessary to bring the drug candidate to worldwide commercialization and with other partners that in-license our technology on a restrictive-market basis. The latter provides access to clinical data which can assist us in making decisions about potential monetization in larger markets.
We believe our orphan oncology drug candidates may meet an established and unmet therapeutic need for a relatively limited population of patients, and products with very high sales potential – benefiting from more favorable price and reimbursement policies.
We advance our drug candidates through a combination of conducting our own in-house research and through the use of contract manufacturing and contract research organizations in order to efficiently manage the Company’s overheads. Continuous pipeline growth and advancement of out-licensed drug candidates is dependent, in part, on several important co-development collaborations and strategic arrangements. Together with our collaborative partners, we are focused on developing our pipeline of next generation bio-therapeutics and novel orphan drugs in oncology based primarily on our PolyXen, OncoHist and Virexxa proprietary technologies.
Our Intellectual Property
We directly or indirectly through our wholly-owned subsidiary, Xenetic U.K., and its wholly-owned subsidiaries, Lipoxen Technologies Limited, or Lipoxen, Xenetic Technologies, Inc. and SymbioTec GmbH, own various U.S. federal trademark registrations and applications, and unregistered trademarks and service marks, including but not limited to Virexxa, OncoHist, PolyXen, ErepoXen, ImuXen, Xemys (formerly MyeloXen), and PulmoXen. Altogether, we directly hold more than 200 issued or allowed patents with 40 in the United States and an additional 161 international patents, and we have approximately 100 pending patent applications worldwide. Since these patents are either held by us or our wholly-owned subsidiaries, we will not distinguish between patents held by us or our subsidiaries in the remainder of this prospectus.
We have drug candidates under various stages of development, each protected by patent and pending patent applications in the U.S. with the United States Patent and Trademark Office, or USPTO, and in certain other developed countries. Generally, patents have a term of 20 years from the earliest priority date (subject to paying all maintenance fees when due). In some instances, patent terms can be increased or decreased, depending on the laws and regulations of the country or jurisdiction that issued the patent, through the filing of a provisional patent application or through such other mechanisms, such as patient term extension (PTE) or supplementary protection certificates (SPC). Our first issued patents are due to begin to expire starting in 2022 with the majority of the existing issued patents expiring between 2027 and 2030.
Our patent strategy is to file patent applications on innovations and improvements in those jurisdictions that comprise the major pharmaceutical markets in the world or locations where a pharmaceutical may be manufactured. These jurisdictions include, but are not limited to the U.S., United Kingdom (U.K.), Australia, Japan, Canada, South Korea, China, Hong Kong, India, Russia and certain other countries in the European Union (E.U.) and Asia, though we do not necessarily file a patent application in each of these jurisdictions for every patent family.
| 6 |
We strive to protect and enhance the proprietary technologies that we believe are important to our business, including seeking and maintaining patents intended to cover our products and compositions, their methods of use and any other inventions that are important to the development of our business. We also rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection.
Our ability to maintain and protect our intellectual property is subject to numerous risks of which you should be aware in making an investment decision. Failure to maintain and protect our intellectual property will have significant impact on our products and technologies. These risks are described more fully in the section entitled "Risk Factors” immediately following this prospectus summary.
Risks Associated with Our Business
Our ability to implement our business strategy is subject to numerous risks of which you should be aware in making an investment decision. These risks are described more fully in the section entitled "Risk Factors" immediately following this prospectus summary.
The single most pressing factor that could cause actual results to differ materially and adversely is our need to raise additional working capital for the purpose of further developing our various drug candidates. We estimate that we have less than three months of working capital as of September 21, 2016 and our future results could differ materially and adversely if we are unable to raise additional working capital. As a result, our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern. Other risk factors include without limitation:
| · | We have a limited operating history, have incurred significant operating losses since our inception and expect to incur significant losses for the foreseeable future. We may never generate any revenue or become profitable or, if we achieve profitability, we may not be able to sustain it. |
| · | Our business currently depends substantially on the success of Virexxa and ErepoXen, which will require significant clinical testing before we can seek regulatory approval and potentially launch commercial sales. If we are unable to obtain regulatory approval for, or successfully commercialize, Virexxa and ErepoXen, our business will be materially harmed. |
| · | Any termination or suspension of, or delays in the commencement or completion of, our planned clinical trials could result in increased costs to us, delay or limit our ability to generate revenue and adversely affect our commercial prospects. |
| · | All of our product candidates are still in preclinical or early-stage clinical development. If we are unable to commercialize our product candidates or if we experience significant delays in obtaining regulatory approval for, or commercializing, any or all of our product candidates, our business will be materially and adversely affected. |
| · | We rely on third-parties to conduct some or all aspects of our product manufacturing, research and preclinical and clinical testing, and these third-parties may not perform satisfactorily. |
| · | Our rights to develop and commercialize our product candidates are subject in part to the terms and conditions of licenses granted to us by other companies. |
| · | Our success depends on our ability to protect our intellectual property and our proprietary technologies. | |
| 7 |
Corporate Update
On July 1, 2016, we issued a convertible promissory note (the “Note”) in the amount of $500,000 to Pharmsynthez, our majority shareholder. In consideration for the promissory note, we issued Pharmsynthez warrants (the “Warrants”) to purchase 50,505 shares of our common stock at the lesser of $6.60 per share and 120% of the price per share in the Company’s next capital raise of at least $15 million. The Note is convertible into shares of our common stock at any time at a conversion price of $4.95 per share (subject to price protection and usual and customary adjustments). The Warrant may be exercised at any time through the five-year anniversary.
Mr. M. Scott Maguire is our Chief Executive Officer. Mr. Maguire’s current annual salary is $505,735 pursuant to his written employment agreement with the Company. Of Mr. Maguire’s 2015 salary amount and 2016 salary amount through today, fifty percent (50%) has been paid in cash and fifty percent (50%) has been deferred and accrued pursuant to an unwritten arrangement between us and Mr. Maguire. On July 1, 2016, we issued a convertible promissory note in the amount of $369,958 and warrants to purchase 37,369 shares of our common stock at the Exercise Price to Mr. Maguire for the deferred salary. We also entered into a Deferred Salary Security Agreement with Mr. Maguire, pursuant to which Mr. Maguire agreed to continue to defer fifty percent (50%) of his salary until the earlier to occur of: (i) the closing of a public offering of our securities concurrent to a NASDAQ listing, or (ii) September 30, 2016 (the “Deferral End Date”). All deferred salary shall become due and payable on the Deferral End Date. As security for the payment of the deferred salary, we granted Mr. Maguire a continuing subordinated security interest in our assets, including all inventory, accounts, accounts receivable, equipment, trademarks, contracts, copyrights and general intangibles.
On August 26 and September 9, 2016 we issued convertible promissory notes in the amount of $178,000 and $322,000, respectively, to Pharmsynthez. The notes are convertible into shares of our common stock at any time at a conversion price of $4.00 per share (subject to price protection and usual and customary adjustments) or may be applied toward the Offering, at the option of Pharmsynthez.
On September 23, 2016, SynBio, one of our largest shareholders exchanged 970,000 shares of common stock in the Company for an equal number of shares of Series A Preferred Stock.
| 8 |
Corporate Information
We are incorporated under the laws of the State of Nevada since August 2011. Our principal executive office is located at 99 Hayden Ave, Suite 230, Lexington, Massachusetts 02421, and our telephone number is (781) 778-7720. Our website address is www.xeneticbio.com. We do not incorporate the information on or accessible through our website into this prospectus, and you should not consider any information on, or that can be accessed through, our website as part of this prospectus.
Implications of Being an Emerging Growth Company
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (JOBS Act). We will remain an emerging growth company until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenue of $1.0 billion or more; (ii) the date on which the market value of our common stock held by non-affiliates exceeds $700.0 million as of any June 30; (iii) the last day of the fiscal year following the fifth anniversary of the completion of our initial public offering, which was declared effective on March 22, 2012; (iv) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; (v) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission, or SEC, which would occur if the market value of our common stock held by non-affiliates exceeded $700.0 million as of the last business day of the second fiscal quarter of such fiscal year or (vi) the date on which we have issued more than $1.0 billion in nonconvertible debt securities during the prior three-year period. An emerging growth company may take advantage of specified reduced reporting requirements and is relieved of certain other significant requirements that are otherwise generally applicable to public companies. As an emerging growth company,
| · | we may avail ourselves of the exemption from the requirement to obtain an attestation and report from our auditors on the assessment of our internal control over financial reporting through fiscal year 2017 pursuant to the Sarbanes-Oxley Act of 2002 (Sarbanes-Oxley); |
| · | we may provide less extensive disclosure about our executive compensation arrangements; and |
| · | we may not require stockholder nonbinding advisory votes on executive compensation or golden parachute arrangements. |
We have irrevocably chosen to opt out of the extended transition periods available to emerging growth companies under the JOBS Act for complying with new or revised accounting standards. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition periods for complying with new or revised accounting standards is irrevocable.
| 9 |
The Offering
| Issuer | Xenetic Biosciences, Inc. |
| Units offered | 500,000 units, consisting of one share of convertible Series B Preferred Stock and a Class
A Warrant to purchase one share of common stock, and 2,000,000 units, consisting of one share of convertible Series
B Preferred Stock and a Class B Warrant to purchase one share of common stock. This prospectus also relates to the offering
of the shares of common stock issuable upon conversion of the Series B Preferred Stock and upon exercise of the Class A and
Class B Warrants. The Class A Warrants will be issued to existing institutional investors of our common stock. All other investors
will receive Class B Warrants.
|
| Description of Series B Preferred Stock | Each unit includes one share of convertible Series B Preferred Stock. The Series B Preferred stock has a liquidation preference and full ratchet price based anti-dilution protection. See the section entitled "Description of Capital Stock—Series B Preferred Stock" beginning on page 116. |
| Conversion rate of Series B Preferred Stock | Each share of Series B Preferred Stock converts into one share of common stock, subject to adjustment as described in this prospectus. |
| Shares of common stock underlying the units | 5,000,000 shares. |
| Description of Warrants | The common stock and warrants will be separately immediately upon issuance. Each warrant will have an exercise price of $ per share and will expire five years from the date of issuance. The Class A Warrants will be exercisable six months after issuance. The Class B Warrants will be exerciseable immediately. A holder (together with its affiliates) may not exercise any portion of the warrant to the extent that the holder would own more than 4.99% of the outstanding common stock after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants. |
| Common Stock to be outstanding after this Offering | 9,024,872 shares. |
Series B Preferred Stock outstanding before this offering |
None. |
| Series B Preferred Stock outstanding after this offering | 2,500,000 shares. |
| Use of Proceeds by Us | We estimate that we will receive net proceeds from this offering of $9.55 million, after deducting underwriting discounts and commissions and estimated offering expenses (assuming none of the warrants issued in this offering are exercised). We expect to use the net proceeds from this offering to fund the research and development of our product candidates, including Virexxa, as well as future development programs, potential in-licensing of products or technology, capital expenditures, working capital, repayment of outstanding convertible promissory notes held by Pharmsynthez and other general corporate purposes. See “Use of Proceeds” for additional information. |
| 10 |
No market for Series B Preferred Stock |
The units will not be certificated and the securities part of such units are immediately separable and will be issued separately in this offering. There is no established public trading market for the Series B Preferred Stock or the warrants issued in this offering, and we do not intend to apply to list such Series B Preferred Stock or warrants on any securities exchange or automated quotation system. |
| Risk Factors | You should carefully read “Risk Factors” in this prospectus for a discussion of factors that you should consider before deciding to invest in our common stock. |
| OTCQB Trading Symbol | “XBIO” |
| Proposed NASDAQ Capital Market Symbol | We have applied to list our common stock on the NASDAQ Capital Market under the symbol “XBIO”. No assurance can be given that our application will be accepted. The closing of this offering is dependent upon the approval for the listing of our common stock on the NASDAQ Capital Market, although this offering will close before such listing. |
The number of shares of our common stock to be outstanding after this offering is based on 9,024,872 shares of our common stock outstanding as of June 30, 2016 and excludes:
| · | 2,500,000 shares of common stock issuable upon the exercise of warrants sold in this offering; | |
| · | 2,500,000 shares of common stock issuable upon the conversion of Series B Preferred Stock sold in this offering; | |
| · | 970,000 shares of common stock issuable upon the conversion of Series A Preferred Stock; |
| · | 671,853 shares of common stock issuable upon the exercise of outstanding stock options at a weighted-average exercise price of $14.99 per share; | |
| · | 758,347 shares of common stock issuable upon the exercise of outstanding warrants at a weighted-average exercise price of $18.21 per share; and |
| · | 681,878 shares of common stock reserved for future issuance under the Equity Incentive Plan (2014 Plan). |
Unless otherwise indicated, all information in this prospectus reflects a 1-for-33 reverse split of our common stock that was effective on June 1, 2016 and assumes and gives effect to the following:
| · | the filing and effectiveness of our amended and restated certificate of incorporation and the adoption of our amended and restated bylaws, each of which will occur immediately prior to the consummation of this offering; | |
| · | On September 23, 2016, SynBio, one of our largest shareholders exchanged 970,000 shares of common stock in the Company for an equal number of shares of Series A Preferred Stock; and |
| · | no issuance or exercise of stock options on or after June 30, 2016. |
| 11 |
SUMMARY CONSOLIDATED FINANCIAL DATA
The following summary consolidated financial data for the six months ended June 30, 2016 and 2015, and the balance sheet data as of June 30, 2016 are derived from our unaudited condensed consolidated financial statements, and for the years ended December 31, 2015 and 2014, and the balance sheet data as of December 31, 2015 and 2014 have been derived from our audited financial statements included elsewhere in this prospectus. You should read this data together with our financial statements and related notes included elsewhere in this prospectus and the information under the captions “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Our historical results are not necessarily indicative of our future results.
| SIX MONTHS ENDED JUNE 30, | YEAR ENDED DECEMBER 31, | |||||||||||||||
| 2016 | 2015 | 2015 | 2014 | |||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
| Operating costs and expenses: | ||||||||||||||||
| Research and development | $ | (2,634,494 | ) | $ | (1,590,823 | ) | $ | (3,434,016 | ) | $ | (6,323,896 | ) | ||||
| IPR&D expense | (39,500,000 | ) | – | – | – | |||||||||||
| General and administrative | (2,980,043 | ) | (1,738,625 | ) | (6,388,000 | ) | (6,600,870 | ) | ||||||||
| Loss from operations | (45,114,537 | ) | (3,329,448 | ) | (9,822,016 | ) | (12,924,766 | ) | ||||||||
| Other Income (Expense): | ||||||||||||||||
| Change in fair value of derivative liability | 1,905,289 | – | (2,125,117 | ) | – | |||||||||||
| Loss on issuance of hybrid debt instrument | (1,584,218 | ) | – | – | – | |||||||||||
| Loss on conversion of debt | (6,187,337 | ) | – | – | – | |||||||||||
| Loss on disposal of subsidiaries | – | – | – | (1,069,675 | ) | |||||||||||
| Other expense | (13,551 | ) | (225,515 | ) | (295,033 | ) | (326,916 | ) | ||||||||
| Interest income | 27 | 1,088 | 1,694 | 18,959 | ||||||||||||
| Interest expense | (348,470 | ) | (2,512 | ) | (266,999 | ) | (4,706 | ) | ||||||||
| (6,228,260 | ) | (226,939 | ) | (2,685,455 | ) | (1,382,338 | ) | |||||||||
| Net Loss | (51,342,797 | ) | (3,556,387 | ) | (12,507,471 | ) | (14,307,104 | ) | ||||||||
| Other Comprehensive Loss from Foreign Currency Translation Adjustment | – | (327,054 | ) | (321,942 | ) | (324,578 | ) | |||||||||
| Total Comprehensive Loss | $ | (51,342,797 | ) | $ | (3,883,441 | ) | $ | (12,829,413 | ) | $ | (14,631,682 | ) | ||||
| Net loss per share of common stock, basic and diluted* | $ | (8.28 | ) | $ | (0.84 | ) | $ | (3.02 | ) | $ | (3.55 | ) | ||||
| Weighted-average shares of common stock outstanding, basic and diluted* | 6,197,776 | 4,221,328 | 4,254,470 | 4,118,062 | ||||||||||||
_______________________
*See Note 2 to our audited financial statements included elsewhere in this prospectus for an explanation of the calculations of our net loss per share—basic and diluted, and the weighted-average shares of common stock outstanding—basic and diluted.
| 12 |
CONSOLIDATED BALANCE SHEETS
| AS OF JUNE 30, 2016 | ||||||||
| Actual Unaudited | Pro Forma (1)(2)(3) | |||||||
| ASSETS | ||||||||
| Current Assets: | ||||||||
| Cash | $ | 240,786 | $ | 9,790,786 | ||||
| Restricted Cash | 66,510 | 66,510 | ||||||
| Prepaid Expenses and Other | 171,199 | 171,199 | ||||||
| Total Current Assets | 478,495 | 10,028,495 | ||||||
| Property and Equipment, Net | 58,471 | 58,471 | ||||||
| Goodwill | 3,283,379 | 3,283,379 | ||||||
| Indefinite-Lived Intangible Assets | 9,243,128 | 9,243,128 | ||||||
| Other Assets | 97,238 | 97,238 | ||||||
| Total Assets | $ | 13,160,711 | $ | 22,710,711 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current Liabilities: | ||||||||
| Accounts Payable | $ | 2,021,205 | 2,021,205 | |||||
| Accrued Expenses | 1,607,125 | 1,607,125 | ||||||
| Other Current Liabilities | 19,647 | 19,647 | ||||||
| Loans Due to Related Parties | 152,529 | 152,529 | ||||||
| Total Current Liabilities | 3,800,506 | 3,800,506 | ||||||
| Deferred Tax Liability | 2,918,518 | 2,918,518 | ||||||
| Other Liabilities | 29,446 | 29,446 | ||||||
| Total Liabilities | 6,748,470 | 6,748,470 | ||||||
| Commitments and Contingent Liabilities (4) | – | – | ||||||
| Stockholders' Equity: | ||||||||
| Preferred Stock, $0.001 par value: 10,000,000 shares authorized; no shares issued and outstanding, actual; 970,000 shares of Series A Preferred issued and outstanding, pro forma | 970 | |||||||
| 0 shares of Series B Preferred issued and outstanding, actual and pro forma | ||||||||
| Common Stock, $0.001 par value; 45,454,546 shares authorized; 9,348,757 shares issued, actual; 10,878,757 shares issued, pro forma; 9,024,872 shares outstanding, actual; 10,554,872 shares outstanding, pro forma | 9,348 | 10,878 | ||||||
| Additional Paid in Capital | 150,905,035 | 160,452,535 | ||||||
| Accumulated Deficit | (139,474,696 | ) | (139,474,696 | ) | ||||
| Accumulated Other Comprehensive Income | 253,734 | 253,734 | ||||||
| Treasury Stock | (5,281,180 | ) | (5,281,180 | ) | ||||
| Total Stockholders' Equity | 6,412,241 | 15,962,241 | ||||||
| Total Liabilities and Stockholders' Equity | $ | 13,160,711 | $ | 22,710,711 | ||||
| (1) | The pro forma gives effect to the sale in this offering of 2,500,000 units at an assumed public offering price of $4.00 per unit, the minimum price for initial listing on the NASDAQ Capital Market, after deducting the underwriting discounts and commissions and estimated offering expenses, and the exchange by SynBio of 970,000 shares of our common stock for 970,000 shares of our Series A Preferred Stock. A $1.00 increase (decrease) in the assumed public offering price of $4.00 per unit would increase (decrease) the amount of current assets, additional paid-in capital, total stockholders’ equity and total capitalization on a pro forma basis by approximately $2.5 million, assuming the number of units offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of one million units offered by us would increase (decrease) current assets, total stockholders’ equity and total capitalization on a pro forma basis by approximately $2.5 million, assuming the assumed public offering price remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will be adjusted based on the actual offering price and other terms of this offering determined at pricing. | |
| (2) | Pro-forma Additional Paid in Capital includes approximately $8.5 million estimated fair value, using the Black-Scholes pricing model, for the expected issuance of an aggregate of 2,500,000 Class A Warrants and Class B Warrants in connection with the offering. | |
| (3) | Assumes the conversion of all the shares of Series B preferred stock sold in the offering into shares of common stock. | |
| (4) | In August 2013, we entered into an agreement to lease office and laboratory space in Lexington, Massachusetts under an operating lease with a commencement date of January 1, 2014 and a termination date of January 31, 2019. With the execution of this lease, we are required to maintain a $66,000 letter of credit as a security deposit, which is classified as a current asset within the consolidated balance sheet. In connection with the Lexington lease, we recorded $90,838 and $76,107 as prepaid rent as of December 31, 2015 and as of June 30, 2016, respectively, with $61,377 and $46,646 recorded as a non-current asset as of December 31, 2015 and as of June 30, 2016, respectively. We also incurred a liability of $89,074 with respect to our contribution to the landlord’s leasehold improvements, of which $47,743 is outstanding as of June 30, 2016, with $29,446 recorded as a non-current liability. This liability is repayable as additional rent expense over the term of the lease and bears interest at 6%. In addition, we leased office space in London, U.K. during 2014 and 2015. The U.K. lease was terminated in March 2015 in accordance with the terms of the lease. |
| 13 |
Risk factors
Before you invest in the units, you should understand the high degree of risk involved. You should carefully consider the following risks and uncertainties and all other information contained in this prospectus before you decide to purchase the units offered by this prospectus. The following risks may adversely impact our business, financial condition, and operating results.
Risks Related to Our Financial Condition and Capital Requirements
We have never been profitable and may never achieve or sustain profitability.
We are a clinical stage biopharmaceutical company with a limited operating history. Pharmaceutical product and technology development is a highly speculative undertaking and involves a substantial degree of risk. To date, we have focused primarily on developing our lead product candidates, ErepoXen, Virexxa and OncoHist, which are currently undergoing clinical development, and PolyXen technology, our biological platform technology, and researching additional product candidates. We have no products approved for commercial sale and have generated only limited revenue to date. We continue to incur significant research and development and other expenses related to our ongoing operations. As a result, we have never been profitable and we may not achieve profitability in the foreseeable future, if at all. Our ability to generate profits in the future will depend on a number of factors, including:
| · | Funding the costs relating to the research and development, regulatory approval, commercialization and sale and marketing of our drug candidates and technologies, in particular, ErepoXen and Virexxa; | |
| · | Market acceptance of our drug candidates and technologies, in particular, ErepoXen and Virexxa; | |
| · | Costs of acquiring and developing new drug candidates and technologies; | |
| · | Ability to bring our drug candidates to market, in particular, ErepoXen and Virexxa; | |
| · | General and administrative costs relating to our operations; | |
| · | Increases in our research and development costs; | |
| · | Charges related to purchases of technology or other assets; | |
| · | Establish, maintain and protect our intellectual property rights; | |
| · | Attract, hire and retain qualified personnel; and | |
| · | Our ability to raise additional capital. |
As of June 30, 2016, we had an accumulated deficit of $139,474,696. We expect to incur additional significant operating losses as we expand our research and development activities and our commercialization, marketing and sales efforts. We may also encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. In addition, because of the numerous risks and uncertainties associated with pharmaceutical product development, including that our current drug candidates may not achieve the clinical endpoints of applicable trials, we are unable to predict the timing or amount of increased expenses, and if or when we will achieve or maintain profitability. If we are unable to generate sufficient revenue from our operations to pay expenses or we are unable to obtain additional financing on commercially reasonable terms, our business, financial condition and results of operations may be materially and adversely affected.
Our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern.
Our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern. As described in their audit report, our auditors have included an explanatory paragraph that states that we have incurred recurring losses and negative cash flows from operations since inception and have an accumulated deficit at June 30, 2016 of $139.5 million and no debt. These matters raise substantial doubt about our ability to continue as a going concern. Our consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. If we cannot continue as a viable entity, our stockholders may lose some or all of their investment in us.
Even if this offering is successful, we will require substantial additional funding to achieve our goals. Failure to obtain this necessary capital when needed on acceptable terms, or at all, may force us to delay, limit or terminate our product development efforts, other operations or commercialization efforts.
We are currently advancing our product candidates through preclinical and clinical development. Developing product candidates is an expensive, risky and lengthy process, and we expect our expenses to increase in connection with our ongoing activities, particularly as we continue the research and development of, continue and initiate clinical trials of, and seek marketing approval for, our product candidates, in particular ErepoXen, Virexxa and OncoHist.
| 14 |
As of June 30, 2016, our cash was $0.2 million. We expect that we will require additional capital to complete clinical trials, obtain regulatory approval for, and to commercialize, our product candidates, including our other preclinical product candidates and our future product candidates. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, third-party funding, marketing and distribution arrangements, as well as other collaborations, strategic alliances and licensing arrangements, or a combination of these approaches. In any event, we will require additional capital to pursue preclinical and clinical activities, pursue regulatory approval for, and to commercialize, our longer term pipeline product candidates. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may negatively impact the holdings or the rights of our stockholders, and the issuance of additional securities, whether equity or debt, by us or the possibility of such issuance may cause the market price of our shares to decline. The incurrence of indebtedness could result in increased fixed payment obligations and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or for specific strategic considerations.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our development programs or the commercialization of any product candidates. We may also be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could harm our business, financial condition and results of operations.
Raising additional capital may cause dilution to our stockholders, including purchasers of units in this offering, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity and debt financings, as well as selectively continuing to enter into collaborations, strategic alliances and licensing arrangements. We do not currently have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, equity interests, including common stock underlying the units, will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder to the extent you have exercised warrants or converted Series B Preferred Stock. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends, and may be secured by all or a portion of our assets.
If we raise funds by selectively continuing to enter into collaborations, strategic alliances or licensing arrangements with third parties, we may have to relinquish additional valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves. If we are unable to raise additional funds through collaborations, strategic alliances or licensing arrangements, we may be required to terminate product development or future commercialization efforts or to cease operations altogether.
We plan to use potential future operating losses and our federal and state net operating loss, or NOL, carryforwards to offset taxable income from revenue generated from operations or corporate collaborations. However, our ability to use NOL carryforwards could be limited as a result of issuance of equity securities.
We plan to use our current year operating losses to offset taxable income from any future revenue generated from operations or corporate collaborations. To the extent that our taxable income exceeds any current year operating losses, we plan to use our NOL carryforwards to offset income that would otherwise be taxable. However, under the Tax Reform Act of 1986, the amount of benefits from our NOL carryforwards may be impaired or limited if we incur a cumulative ownership change of more than 50%, as interpreted by the U.S. Internal Revenue Service, over a three-year period. As a result, our use of federal NOL carryforwards could be limited by the provisions of Section 382 of the U.S. Internal Revenue Code of 1986, as amended, depending upon the timing and amount of additional equity securities that we issue. In addition, we have not performed an analysis of limitations, and we may have experienced an ownership change under Section 382 as a result of past financings. State NOL carryforwards may be similarly limited. Any such disallowances may result in greater tax liabilities than we would incur in the absence of such a limitation and any increased liabilities could adversely affect our business, results of operations, financial condition and cash flow.
| 15 |
Our liquidity and operating plans will be negatively affected if we are unable to list our common stock on the NASDAQ Capital Markets.
We have applied for our common stock to be listed on the NASDAQ Capital Markets. No assurance can be given that our application will be approved. In the event that the Company is unable to cause a listing of its securities on the NASDAQ Capital Markets, then, Pharmsynthez shall loan to the Company up to $6.0 million on essentially similar terms as existing 10% Convertible Promissory Notes issued by the Company to Pharmsynthez. This outcome would require the Company to seek additional financing and/or defer certain research and development activities in order to meet its financial obligations over the next 12 months.
Risks Related to the Discovery and Development of our Pharmaceutical Products
We are an early stage company in the business of developing pharmaceutical products including drug candidates and technologies. Given the uncertainty of such development our business operations may never fully materialize and create value for investors.
| · | We currently do not have any products that have gained marketing approval. We have invested substantially all of our efforts and financial resources developing ErepoXen, OncoHist and Virexxa, our lead candidates that are in the early stages of development. Our revenues to date consist primarily of collaboration revenue from a single partner and not from product sales or royalties. Our ability to generate product revenues, which may not occur for several years, if ever, will depend on the successful development and eventual commercialization of our drug candidates. We currently generate no revenues from sales of any drugs, and we may never be able to develop or commercialize a marketable drug. Each of our product candidates will require development, management of development and manufacturing activities, marketing approval in multiple jurisdictions, obtaining manufacturing supply, building of a commercial organization, substantial investment and significant marketing efforts before we generate any revenues from drug sales. We have not yet demonstrated an ability to successfully overcome many of the risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the pharmaceutical area. For example, to execute our business plan, we will need to successfully: | |
| · | Execute development activities for our product candidates, including successful enrollment in and completion of clinical trials; | |
| · | Obtain required marketing approvals for the development and commercialization of our product candidates; | |
| · | Obtain and maintain patent and trade secret protection or regulatory exclusivity for our product candidates; | |
| · | Protect, leverage and expand our intellectual property portfolio; | |
| · | Establish and maintain clinical and commercial manufacturing capabilities or make arrangements with third-party manufacturers for clinical and commercial manufacturing; | |
| · | Build and maintain robust sales, distribution and marketing capabilities, either on our own or in collaboration with strategic partners, if our product candidates are approved; | |
| · | Gain acceptance for our product candidates, if approved, by patients, the medical community and third party payors; | |
| · | Effectively compete with other therapies; | |
| · | Obtain and maintain healthcare coverages and adequate reimbursement; | |
| · | Maintain a continued acceptable safety profile for our product candidates following approval; | |
| · | Develop and maintain any strategic relationships we elect to enter into, if any; | |
| · | Enforce and defend intellectual property rights and claims; and | |
| · | Manage our spending as costs and expenses increase due to preclinical development, clinical trials, marketing approvals and commercialization. |
We may find it difficult to enroll patients in our clinical studies, which could delay or prevent clinical studies of our pharmaceutical products.
Identifying and qualifying patients to participate in clinical studies of our pharmaceutical products is critical to our success. The timing of our clinical studies depends on the speed at which we can recruit patients to participate in testing our pharmaceutical products. We may experience delays. If patients are unwilling to participate in our clinical studies because of negative publicity from adverse events in the biophamaceutical industries or for other reasons, including competitive clinical studies for similar patient populations, the timeline for recruiting patients, conducting studies and obtaining regulatory approval of potential products may be delayed. These delays could result in increased costs, delays in advancing our product development, delays in testing the effectiveness of our technology or termination of the clinical studies altogether.
| 16 |
We may not be able to identify, recruit and enroll a sufficient number of patients, or those with required or desired characteristics to achieve diversity in a study, to complete our clinical studies in a timely manner. Patient enrollment is affected by factors including:
| · | Severity of the disease under investigation; | |
| · | Real or perceived availability of alternative treatments; | |
| · | Size and nature of the patient population; | |
| · | Eligibility criteria for and design of the trial in question; | |
| · | Perceived risks and benefits of the product candidate under study; | |
| · | Proximity and availability of clinical sites for prospective patients; | |
| · | Ongoing clinical trials of potentially competitive agents; | |
| · | Physicians’ and patients’ perceptions as to the potential advantages of our product candidates being studied in relation to available therapies or other products under development; | |
| · | Our CRO’s and our trial sites’ efforts to facilitate timely enrollment in clinical trials; | |
| · | Patient referral practices of physicians; and | |
| · | The need to monitor patients and collect patient data adequately during and after treatment. |
We may not be able to initiate or continue clinical studies if we cannot enroll a sufficient number of eligible patients to participate in the clinical studies required by the FDA or other regulatory agencies. Our ability to successfully initiate, enroll and complete a clinical study in any foreign country is subject to numerous risks unique to conducting business in foreign countries, including:
| · | Difficulty in establishing or managing relationships with contract research organizations, and physicians; | |
| · | Different standards for the conduct of clinical studies; | |
| · | Our inability to locate qualified local consultants, physicians and partners; and | |
| · | The potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of pharmaceutical and biotechnology products and treatment. |
If we have difficulty enrolling a sufficient number of patients to conduct our clinical studies as planned, we may need to delay, limit or terminate ongoing or planned clinical studies, any of which would have an adverse effect on our business.
We may encounter substantial delays in commencement, enrollment or completion of our clinical trials or we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities, which could prevent us from commercializing our current and future product candidates on a timely basis, if at all.
Before obtaining marketing approval from regulatory authorities for the sale of our current and future product candidates, we must conduct extensive clinical trials to demonstrate the safety and efficacy of the product candidates. We cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical studies can occur at any stage of testing. Events that may prevent successful or timely completion of clinical development include:
| · | Delays in reaching a consensus with regulatory agencies on study design; | |
| · | Delays in reaching agreement on acceptable terms with prospective CROs and clinical study sites; | |
| · | Delays in obtaining required Institutional Review Board, or Independent Ethics Committee approval at each clinical study site; | |
| · | Delays in recruiting suitable patients to participate in our clinical studies; | |
| · | Imposition of a clinical hold by regulatory agencies, including after an inspection of our clinical study operations or study sites; | |
| · | Failure by our CROs, other third-parties or us to adhere to clinical study requirements; | |
| · | Failure to perform in accordance with the FDA’s good clinical practices (GCP), or applicable regulatory requirements in other countries; | |
| · | Delays in the testing, validation, manufacturing and delivery of our product candidates to the clinical sites; | |
| · | Delays in having patients complete participation in a study or return for post-treatment follow-up; | |
| · | Clinical study sites or patients dropping out of a study; | |
| · | Occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits; or | |
| · | Changes in regulatory requirements and guidance that require amending or submitting new clinical protocols. |
| 17 |
Any inability to successfully complete preclinical studies and clinical trials could result in additional costs to us or impair our ability to generate revenues from product sales, regulatory and commercialization milestones and royalties. In addition, if we make manufacturing or formulation changes to our product candidates, we may need to conduct additional studies to bridge our modified product candidates to earlier versions. Clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates and may harm our business, financial condition, results of operations and prospects.
If the results of our clinical studies are inconclusive or if there are safety concerns or adverse events associated with our pharmaceutical products, we may:
| · | Be delayed in obtaining marketing approval or licenses for our product candidates, if at all; | |
| · | Obtain approval for indications or patient populations that are not as broad as intended or desired; | |
| · | Obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; | |
| · | Be subject to changes with the way the product is administered; | |
| · | Be required to perform additional clinical studies to support approval or be subject to additional post-marketing testing requirements; | |
| · | Have regulatory authorities withdraw their approval of the product or impose restrictions on its distribution in the form of a modified risk evaluation and mitigation strategy; | |
| · | Be subject to the addition of labeling statements, such as warnings or contraindications; | |
| · | Be sued; or | |
| · | Experience damage to our reputation. |
As described above, any of these events could prevent us from achieving or maintaining market acceptance of our pharmaceutical products and impair our ability to generate revenues.
Clinical trials may fail to demonstrate the safety and efficacy of our pharmaceutical drug candidates and could prevent or significantly delay regulatory approval.
Before receiving NDA or BLA approval to commercialize a drug candidate, we must demonstrate to the FDA, with substantial evidence from well controlled clinical trials, that the drug candidate is both safe and effective or the biologics is safe, pure and potent. If these trials or future clinical trials are unsuccessful, our business and reputation would be harmed and our stock price would most likely be adversely affected.
Clinical failure can occur at any stage of clinical development. Clinical trials may produce negative or inconclusive results, and we or any of our current and future collaborators may decide, or regulators may require us, to conduct additional clinical or preclinical testing. We will be required to demonstrate with substantial evidence through well-controlled clinical trials that our product candidates are as safe and effective for use in a specific patient population as the respective reference products before we can seek regulatory approvals for their commercial sale. Success in early clinical trials does not mean that future larger registration clinical trials will be successful because product candidates in later-stage clinical trials may fail to demonstrate equivalent safety and efficacy to the satisfaction of the FDA and foreign regulatory agencies despite having progressed through initial clinical trials. Product candidates that have shown promising results in early clinical trials may still fail in subsequent confirmatory clinical trials. Similarly, the outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. A number of companies in the pharmaceutical industry, including those with greater resources and experience than us, have suffered significant setbacks in advanced clinical trials, even after obtaining promising results in earlier clinical trials.
In addition, the design of a clinical trial can determine whether its results will support approval of a product and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. We may be unable to design and execute a clinical trial to support regulatory approval. In some instances, there can be significant variability in safety or efficacy results between different trials of the same product candidate due to numerous factors, including but not limited to changes in trial protocols, differences in size and type of the patient populations, adherence to the dosing regimen and the rate of dropout among clinical trial participants.
Because of these risks, the research and development efforts of our collaborative partners may not result in any commercially viable products. If a significant portion of these development efforts is not successfully completed or, if required regulatory approvals are not obtained by our partners, or any approved products are not commercially successful, we are not likely to generate significant revenues or become profitable.
| 18 |
Even if we complete the necessary preclinical and clinical studies, we cannot predict when or if we will obtain regulatory approval to commercialize a drug candidate or the approval may be for a more narrow indication than we expect.
A drug candidate cannot be commercialized until the appropriate regulatory authorities have reviewed and approved the product candidate. Even if our drug candidates demonstrate safety and efficacy in clinical studies, the regulatory agencies may not complete their review processes in a timely manner, or we may not be able to obtain regulatory approval. Additional delays may result if an FDA Advisory Committee or other regulatory advisory group or authority recommends non-approval or restrictions on approval. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory agency policy during the period of product development, clinical studies and the review process. Regulatory agencies also may approve a drug candidate for fewer or more limited indications than requested or may grant approval subject to the performance of post-marketing studies. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our drug candidates.
Even if we obtain regulatory approval for a drug candidate, our drug candidate will remain subject to regulatory scrutiny.
If our product candidates are approved, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping, conduct of post-marketing studies and submission of safety, efficacy and other post-market information, including both federal and state requirements in the United States and requirements of comparable foreign regulatory authorities.
Manufacturers and manufacturing facilities are required to comply with extensive FDA, and comparable foreign regulatory authority, requirements, including ensuring that quality control and manufacturing procedures conform to current Good Manufacturing Practices, or cGMP, regulations. As such, we will be subject to continual review and inspections to assess compliance with cGMP and adherence to commitments made in any non-disclosure agreement, BLA or marketing authorization application, or MAA. Accordingly, we and our collaborators and suppliers must continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production and quality control.
Any regulatory approvals that we or our collaboration partners receive for our product candidates may be subject to limitations on the approved indicated uses for which the product may be marketed or to the conditions of approval or may contain requirements for potentially costly additional clinical trials and surveillance to monitor the safety and efficacy of the product candidate. We will be required to report certain adverse reactions and production problems, if any, to the FDA and comparable foreign regulatory authorities. Any new legislation addressing drug safety issues could result in delays in product development or commercialization or increased costs to assure compliance. We will have to comply with requirements concerning advertising and promotion for our products. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved label. As such, we are not allowed to promote our products for indications or uses for which they do not have approval. If our product candidates are approved, we must submit new or supplemental applications and obtain approval for certain changes to the approved products, product labeling or manufacturing process. We could also be asked to conduct post-marketing clinical trials to verify the safety and efficacy of our products in general or in specific patient subsets. An unsuccessful post-marketing study or failure to complete such a study could result in the withdrawal of marketing approval.
If a regulatory agency discovers previously unknown problems with an approved product, such as adverse events of unanticipated severity or frequency or problems with our manufacturing facilities or disagrees with the promotion, marketing or labeling of a product, such regulatory agency may impose restrictions on that product or us, including requiring withdrawal of the product from the market. If we fail to comply with applicable regulatory requirements, a regulatory agency or enforcement authority may, among other things:
| · | Issue untitled and warning letters; | |
| · | Impose civil or criminal penalties; | |
| · | Suspend or withdraw regulatory approval or revoke a license; | |
| · | Suspend any of our ongoing clinical trials; | |
| · | Refuse to approve pending applications or supplements to approved applications submitted by us; | |
| · | Impose restrictions on our operations, including closing our manufacturing facilities; or | |
| · | Seize or detain products or require a product recall. |
| 19 |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenue from our products. If regulatory sanctions are applied or if regulatory approval is withdrawn, the value of our company and our operating results will be negatively impacted.
The commercial success of any current or future pharmaceutical products will depend upon the degree of market acceptance by physicians, patients, third-party payors and others in the medical community.
Even with the requisite approvals, the commercial success of our pharmaceutical products will depend in part on the medical community, patients, and third-party payors accepting our pharmaceutical products as medically useful, cost-effective, and safe. Any pharmaceutical product that we or our partners bring to the market may not gain market acceptance by physicians, patients, third-party payors and others in the medical community. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of these pharmaceutical products, if approved for commercial sale, will depend on a number of factors, including:
| · | The effectiveness of our approved drug candidates as compared to currently available products; | |
| · | Patient willingness to adopt our approved drug candidates in place of current therapies; | |
| · | Our ability to provide acceptable evidence of safety and efficacy; | |
| · | Relative convenience and ease of administration; | |
| · | The prevalence and severity of any adverse side effects; | |
| · | Restrictions on use in combination with other products; | |
| · | Availability of alternative treatments; | |
| · | Pricing and cost-effectiveness assuming either competitive or potential premium pricing requirements, based on the profile of our drug candidates and target markets; | |
| · | Effectiveness of our or our partners’ sales and marketing strategy; | |
| · | Our ability to obtain Sufficient third-party coverage or reimbursement; and | |
| · | Potential product liability claims. |
Even if a potential product displays a favorable efficacy and safety profile in preclinical and clinical studies, market acceptance of the product will not be known until after it is launched. Our efforts to educate the medical community and third-party payors on the benefits of the pharmaceutical products may require significant resources and may never be successful.
The commercial potential of a pharmaceutical candidate in development is difficult to predict. If the market size for a new drug candidate or technology is significantly smaller than we anticipate, it could significantly and negatively impact our revenue, results of operations and financial condition.
It is very difficult to estimate the commercial potential of pharmaceutical products due to important factors such as safety and efficacy compared to other available technologies or treatments, including changing standards of care, third-party payor reimbursement standards, patient and physician preferences, the availability of competitive alternatives that may emerge either during the long drug development process or after commercial introduction, and the availability of generic versions of our successful drug candidates following approval by government health authorities based on the expiration of regulatory exclusivity or our inability to prevent generic versions from coming to market by asserting our patents. If due to these factors, or others, the market potential for a pharmaceutical product is lower than we anticipated, it could significantly and negatively impact the commercial terms of any collaboration partnership potential for such pharmaceutical product or, if we have already entered into a collaboration for such pharmaceutical product, the revenue potential from royalty and milestone payments could be significantly diminished which would negatively impact our business, financial condition and results of operations.
Failure to obtain or maintain adequate coverage and reimbursement for our drug candidates, if approved, could limit our ability to market those products and decrease our ability to generate revenue.
The success of our product candidates, if approved, depends on the availability of adequate coverage and reimbursement from third-party payors. In addition, because our product candidates represent new approaches to the treatment of certain diseases, we cannot be sure that coverage and reimbursement will be available for, or accurately estimate the potential revenue from, our product candidates or assure that coverage and reimbursement will be available for any product that we may develop.
| 20 |
Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Adequate coverage and reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors are critical to new product acceptance.
Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which drugs and treatments they will cover and the amount of reimbursement. Coverage and reimbursement by a third-party payor may depend upon a number of factors, including the third-party payor’s determination that use of a product is:
| · | A covered benefit under its health plan; | |
| · | Safe, effective and medically necessary; | |
| · | Appropriate for the specific patient; | |
| · | Cost-effective; and | |
| · | Neither experimental nor investigational. |
In the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors. As a result, obtaining coverage and reimbursement approval of a product from a government or other third-party payor is a time-consuming and costly process that could require us to provide to each payor supporting scientific, clinical and cost-effectiveness data for the use of our products on a payor-by-payor basis, with no assurance that coverage and adequate reimbursement will be obtained. Even if we obtain coverage for a given product, the resulting reimbursement payment rates might not be adequate for us to achieve or sustain profitability or may require co-payments that patients find unacceptably high. Additionally, third-party payors may not cover, or provide adequate reimbursement for, long-term follow-up evaluations required following the use of our gene-modifying products. Patients are unlikely to use our product candidates unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our product candidates. There is significant uncertainty related to insurance coverage and reimbursement of newly approved products. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement for our product candidates.
Moreover, increasing efforts by governmental and third-party payors in the United States and abroad to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our product candidates. We expect to experience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare, the increasing influence of health maintenance organizations, cost containment initiatives and additional legislative changes.
We intend to seek approval to market our product candidates in both the United States and in select foreign jurisdictions. If we obtain approval in one or more foreign jurisdictions for our product candidates, we will be subject to rules and regulations in those jurisdictions. In some foreign countries, the pricing of pharmaceutical products is subject to governmental control and other market regulations which could put pressure on the pricing and usage of our product candidates. In these countries, pricing negotiations with governmental authorities can take considerable time after obtaining marketing approval of a product candidate. In addition, market acceptance and sales of our product candidates will depend significantly on the availability of adequate coverage and reimbursement from third-party payors for our product candidates and may be affected by existing and future health care reform measures.
We may use our financial and human resources to pursue a particular research program or product candidate and fail to capitalize on programs or product candidates that may be more profitable or for which there is a greater likelihood of success.
Because we have limited resources, we may forego or delay pursuit of opportunities with certain programs or product candidates or for indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs for product candidates may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through strategic collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate, or we may allocate internal resources to a product candidate in a therapeutic area in which it would have been more advantageous to enter into a partnering arrangement.
| 21 |
We may not be successful in our efforts to identify or discover additional pharmaceutical products.
The success of our business depends primarily upon our ability to identify and develop pharmaceutical products. Although our existing pharmaceutical products are currently in clinical development, our research programs may fail to identify other potential pharmaceutical products for clinical development for a number of reasons. Our research methodology may be unsuccessful in identifying potential pharmaceutical products or our potential pharmaceutical products may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval.
If any of these events occur, we may be forced to abandon our development efforts for a program or programs, which would have a material adverse effect on our business and could potentially cause us to cease operations. Research programs to identify new pharmaceutical products require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or pharmaceutical products that ultimately prove to be unsuccessful.
We may fail to obtain orphan drug designations from the FDA for our drug candidates, and even if we obtain such designations, we may be unable to maintain the benefits associated with orphan drug designation, including the potential for market exclusivity.
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug or biologic intended to treat a rare disease or condition, which is defined as one occurring in a patient population of fewer than 200,000 in the United States, or a patient population greater than 200,000 in the United States where there is no reasonable expectation that the cost of developing the drug or biologic will be recovered from sales in the United States. In the United States, orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages and user-fee waivers. In addition, if a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications, including a full NDA or BLA, to market the same drug or biologic for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity or where the manufacturer is unable to assure sufficient product quantity.
OncoHist for AML and Virexxa for endometrial cancer have orphan designation in the U.S. While we have not obtained nor have we sought to obtain additional orphan designations for any product candidate, we believe our products candidates could qualify for additional orphan drug designations for additional indications. We may seek to obtain orphan drug designation for our product candidates for any qualifying indications they may be approved for in the future. Even if we obtain such designations, we may not be the first to obtain marketing approval of our product candidate for the orphan-designated indication due to the uncertainties associated with developing pharmaceutical products. In addition, exclusive marketing rights in the United States may be limited if we seek approval for an indication broader than the orphan-designated indication or may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition. Further, even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs with different active moieties can be approved for the same condition. Even after an orphan product is approved, the FDA can subsequently approve the same drug with the same active moiety for the same condition if the FDA concludes that the later drug is safer, more effective or makes a major contribution to patient care. Orphan drug designation neither shortens the development time or regulatory review time of a drug, nor gives the drug any advantage in the regulatory review or approval process. In addition, while we may seek orphan drug designation for our product candidates, we may never receive such designations.
The market opportunities for our product candidates may be limited to those patients who are ineligible for or have failed prior treatments and may be small.
Cancer therapies are sometimes characterized as first line, second line or third line, and the FDA often approves new therapies initially only for third line use. When cancer is detected early enough, first line therapy is sometimes adequate to cure the cancer or prolong life without a cure. Whenever first line therapy, usually chemotherapy, hormone therapy, surgery or a combination of these, proves unsuccessful, second line therapy may be administered. Second line therapies often consist of more chemotherapy, radiation, antibody drugs, tumor targeted small molecules or a combination of these. Third line therapies can include bone marrow transplantation, antibody and small molecule targeted therapies, more invasive forms of surgery and new technologies. In markets with approved therapies, we expect to initially seek approval of our product candidates as a later stage therapy for patients who have failed other approved treatments. Subsequently, for those drugs that prove to be sufficiently beneficial, if any, we would expect to seek approval as a second line therapy and potentially as a first line therapy, but there is no guarantee that our drug candidates, even if approved, would be approved for second line or first line therapy. In addition, we may have to conduct additional clinical trials prior to gaining approval for second line or first line therapy.
| 22 |
Our projections of both the number of people who have the cancers we are targeting, as well as the subset of people with these cancers in a position to receive later stage therapy and who have the potential to benefit from treatment with our product candidates, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations or market research and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these cancers. The number of patients may turn out to be lower than expected. In addition, the potentially addressable patient population for our product candidates may be limited or may not be amenable to treatment with our product candidates. Even if we obtain significant market share for our product candidates, we may never achieve profitability without obtaining regulatory approval for additional indications, including use as a first or second line therapy.
Risks Related to Our Reliance on Third-Parties
If conflicts arise between us and our collaborators or strategic partners, these parties may act in their self-interest, which may limit our ability to implement our strategies.
If conflicts arise between our corporate or academic collaborators or strategic partners and us, the other party may act in its self-interest, which may limit our ability to implement our strategies. Some of our academic collaborators and strategic partners are conducting multiple product development efforts within each area that is the subject of the collaboration with us. Our collaborators or strategic partners, however, may develop, either alone or with others, products in related fields that are competitive with the products or potential products that are the subject of these collaborations. Competing products, either developed by the collaborators or strategic partners or to which the collaborators or strategic partners have rights, may result in the withdrawal of partner support for our product candidates.
Some of our collaborators or strategic partners could also become our competitors in the future. Our collaborators or strategic partners could develop competing products, preclude us from entering into collaborations with their competitors, fail to obtain timely regulatory approvals, terminate their agreements with us prematurely, or fail to devote sufficient resources to the development and commercialization of products. Any of these developments could harm our product development efforts.
In addition to our own clinical trials, we expect to rely on third-parties to conduct, supervise and monitor our clinical studies, and if these third-parties perform in an unsatisfactory manner, it may harm our business.
In addition to our own clinical trials, we expect to rely on CROs, clinical investigators and clinical study sites to ensure our clinical studies are conducted properly and on time. While we will have agreements governing their activities, we will have limited influence over their actual performance. We will control only certain aspects of our CROs’ activities. Nevertheless, we will be responsible for ensuring that each of our clinical studies is conducted in accordance with the applicable protocol, legal, and regulatory requirements and scientific standards, and our reliance on the CROs does not relieve us of our regulatory responsibilities.
We, clinical investigators and our CROs are required to comply with the FDA’s GCPs for conducting, recording and reporting the results of clinical trials to assure that the data and reported results are credible and accurate and that the rights, integrity and confidentiality of clinical trial participants are protected. The FDA enforces these GCPs through periodic inspections of study sponsors, principal investigators and clinical trial sites. If we or our CROs or the clinical investigators fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA may require us to perform additional clinical trials before approving any marketing applications. Upon inspection, the FDA may determine that our clinical trials did not comply with GCPs. In addition, our future clinical trials will require a sufficient number of test subjects to evaluate the safety and efficacy of our product candidates. Accordingly, if our CROs or clinical investigators fail to comply with these regulations or fail to recruit a sufficient number of patients, we may be required to repeat such clinical trials, which would delay the regulatory approval process.
Our CROs are not our employees, and we are therefore unable to directly monitor whether or not they devote sufficient time and resources to our clinical and nonclinical programs, which must be conducted in accordance with GCPs and GLPs, respectively. These CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical studies or other drug development activities that could harm our competitive position. If our CROs do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements, or for any other reasons, our clinical studies may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize our pharmaceutical products. As a result, our financial results and the commercial prospects for our pharmaceutical products would be harmed, our costs could increase, and our ability to generate revenues could be delayed.
| 23 |
We may also rely on other third-parties to store and distribute our products for any clinical studies that we may conduct. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our pharmaceutical products or commercialization of our products, if approved, producing additional losses and depriving us of potential product revenue.
Our collaborators or strategic partners may decide to adopt alternative technologies or may be unable to develop commercially viable products with our technology, which would negatively impact our revenues and our strategy to develop these products.
Our collaborators or strategic partners may adopt alternative technologies, which could decrease the marketability of our products. Additionally, because our current or future collaborators or strategic partners are likely to be working on more than one development project, they could choose to shift their resources to projects other than those they are working on with us. If they do so, this would delay our ability to test our technology and would delay or terminate the development of potential products based on our platforms. Further, our collaborators and strategic partners may elect not to develop products arising out of our collaborative and strategic partnering arrangements or to devote sufficient resources to the development, manufacturing, marketing or sale of these products. The failure to develop and commercialize a product candidate pursuant to our agreements with our current or future collaborator would prevent us from receiving future milestone and royalty payments which would negatively impact our revenues.
We may seek to establish additional collaborations and, if we are not able to establish them on commercially reasonable terms, we may have to alter our development and commercialization plans.
Our product candidate development programs and the potential commercialization of our product candidates will require substantial additional cash to fund expenses. For some of our product candidates, we may decide to collaborate with additional pharmaceutical and biotechnology companies for the development and potential commercialization of those product candidates.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for any additional collaborations will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by FDA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing drugs, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our product candidate. The terms of any additional collaborations or other arrangements that we may establish may not be favorable to us.
We may also be restricted under existing collaboration agreements from entering into future agreements on certain terms with potential collaborators. Collaborations are complex and time-consuming to negotiate and document. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
We may not be able to negotiate additional collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of the product candidate for which we are seeking to collaborate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop our product candidates or bring them to market and generate product revenue.
| 24 |
If we enter into one or more collaborations, we may be required to relinquish important rights to and control over the development of our product candidates or otherwise be subject to unfavorable terms.
Any future collaborations we enter into could subject us to a number of risks, including:
| · | We may not be able to control the amount and timing of resources that our collaborators devote to the development or commercialization of our product candidates; | |
| · | Collaborators may delay clinical trials, provide insufficient funding, terminate a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new version of a product candidate for clinical testing; | |
| · | Collaborators may not pursue further development and commercialization of products resulting from the strategic partnering arrangement or may elect to discontinue research and development programs; | |
| · | Collaborators may not commit adequate resources to the marketing and distribution of our product candidates, limiting our potential revenues from these products; | |
| · | Disputes may arise between us and our collaborators that result in the delay or termination of the research, development or commercialization of our product candidates or that result in costly litigation or arbitration that diverts management’s attention and consumes resources; | |
| · | Collaborators may experience financial difficulties; | |
| · | Collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in a manner that could jeopardize or invalidate our proprietary information or expose us to potential litigation; | |
| · | Business combinations or significant changes in a collaborator’s business strategy may also adversely affect a collaborator’s willingness or ability to complete its obligations under any arrangement; | |
| · | Collaborators could decide to move forward with a competing product candidate developed either independently or in collaboration with others, including our competitors; and | |
| · | Collaborators could terminate the arrangement or allow it to expire, which would delay the development and may increase the cost of developing our product candidates. |
Our contract manufacturers are subject to significant regulation with respect to manufacturing our products. The manufacturing facilities on which we rely may not continue to meet regulatory requirements and have limited capacity.
We currently have relationships with a limited number of suppliers for the manufacturing of our pharmaceutical products. Each supplier may require licenses to manufacture components if such processes are not owned by the supplier or in the public domain and we may be unable to transfer or sublicense the intellectual property rights we may have with respect to such activities.
All entities involved in the preparation of pharmaceutical products for clinical studies or commercial sale, including our existing contract manufacturers for our product candidates, are subject to extensive regulation. Components of a finished pharmaceutical product approved for commercial sale or used in late-stage clinical studies must be manufactured in accordance with cGMP. These regulations govern manufacturing processes and procedures (including record keeping) and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Poor control of production processes can lead to the introduction of adventitious agents or other contaminants, or to inadvertent changes in the properties or stability of our pharmaceutical products that may not be detectable in final product testing. Our contract manufacturers must supply all necessary documentation in support of an NDA or BLA on a timely basis and must adhere to the FDA’s good laboratory practices (GLP), and cGMP regulations enforced by the FDA through its facilities inspection program. The facilities and quality systems of some or all of our third-party contractors must pass a pre-approval inspection for compliance with the applicable regulations as a condition of regulatory approval of our pharmaceutical products or any of our other potential products. In addition, the regulatory authorities may, at any time, audit or inspect a manufacturing facility involved with the preparation of our pharmaceutical products or our other potential products or the associated quality systems for compliance with the regulations applicable to the activities being conducted. If these facilities do not pass a pre-approval plant inspection, FDA approval of the products will not be granted.
The regulatory authorities also may, at any time following approval of a product for sale, audit the manufacturing facilities of our third-party contractors. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time-consuming for us or a third-party to implement and that may include the temporary or permanent suspension of a clinical study or commercial sales or the temporary or permanent closure of a facility. Any such remedial measures imposed upon third-parties with whom we contract could materially harm our business.
| 25 |
If our third-party manufacturers fail to maintain regulatory compliance, the FDA can impose regulatory sanctions including, among other things, refusal to approve a pending application for a product candidate, or revocation of a pre-existing approval. As a result, our business, financial condition and results of operations may be materially harmed.
Additionally, if supply from one approved manufacturer is interrupted, there could be a significant disruption in commercial supply. The number of manufacturers with the necessary manufacturing capabilities is limited. In addition, an alternative manufacturer would need to be qualified through an NDA or BLA supplement which could result in further delay. The regulatory agencies may also require additional studies if a new manufacturer is relied upon for commercial production. Switching manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines.
These factors could cause the delay of clinical studies, regulatory submissions, required approvals or commercialization of our pharmaceutical products, cause us to incur higher costs and prevent us from commercializing our products successfully. Furthermore, if our suppliers fail to meet contractual requirements, and we are unable to secure one or more replacement suppliers capable of production at a substantially equivalent cost, our clinical studies may be delayed or we could lose potential revenue.
We have no manufacturing, sales, marketing or distribution capabilities, and we may have to invest significant resources to develop these capabilities.
We have no internal manufacturing capabilities. As a result, for manufacturing we depend on third-party manufacturers, including Kevelt, Pharmsynthez and the Serum Institute, which in turn may rely upon third-parties to manufacture our products. Although our strategy is based on leveraging the ability of collaboration partners to develop and manufacture our products for commercialization in the pharmaceutical marketplace, we will be dependent on collaborations with drug development and manufacturing collaborators. If we are not able to maintain existing collaborative arrangements or establish new arrangements on commercially acceptable terms, we would be required to undertake product manufacturing and development activities at our own expense. This would increase our capital requirements or require us to limit the scope of our development activities. Moreover, we have limited or no experience in conducting full scale bioequivalence or other clinical studies, preparing and submitting regulatory applications, and distributing and marketing pharmaceutical products and as such we are reliant on contract parties for such efforts. We may not be able to enter into collaborations or hire consultants or external service providers to assist us in sales, marketing and distribution functions on acceptable financial terms or at all.
If any of our developmental collaborators breach or terminate their agreements with us or otherwise fail to conduct their collaborative activities in a timely manner, the pre-clinical and/or clinical development and/or commercialization of our pharmaceutical products will be delayed and we would be required to devote additional resources to product development and commercialization or terminate certain development programs. Also a license relationship may be terminated at the discretion of our collaborator, or at the end of contract terms, and in some cases with only limited notice to us. The termination of the collaborative arrangement could have a material adverse effect on our business, financial condition and results of operations. There also can be no assurance that disputes will not arise with respect to the ownership of rights to any technology developed with third-parties. These and other possible disagreements with collaborators could lead to delays in the development or commercialization of our pharmaceutical products or could result in litigation or arbitration, which could be time consuming and expensive and could have a material adverse effect on our business, financial condition and results of operations. Even if we decide to perform clinical trials, sales, marketing and distribution functions ourselves, we could face a number of additional related risks, including:
| · | we may not be able to attract clinical investigators and build effective clinical trials, or a solid marketing department or sales force; |
| · | the cost of establishing an internal clinical trials program, marketing department or sales force may exceed our available financial resources and the revenue generated by Virexxa, if approved, or any other pharmaceutical products that we may develop, in-license or acquire; and |
| · | our direct sales and marketing efforts may not be successful. |
| 26 |
Any failure to perform such activities could have a material adverse effect on our business, financial condition and results of our operations.
Our reliance on third-parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we rely on third-parties to manufacture our pharmaceutical products, and because we collaborate with various organizations and academic institutions on the development of our pharmaceutical products, we must, at times, share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third-parties to use or disclose our confidential information, such as trade secrets. Despite the contractual provisions employed when working with third-parties, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure would impair our competitive position and may have a material adverse effect on our business.
In addition, these agreements typically restrict the ability of our collaborators, advisors, employees and consultants to publish data potentially relating to our trade secrets. Our academic collaborators typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. In other cases, publication rights are controlled exclusively by us, although in some cases we may share these rights with other parties. We may also conduct joint research and development programs that may require us to share trade secrets under the terms of our research and development partnerships or similar agreements. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication. A competitor’s discovery of our trade secrets would impair our competitive position and have an adverse impact on our business.
Risks Related to Our Intellectual Property
If we fail to adequately protect or enforce our intellectual property rights, we may be unable to operate effectively.
Our success and ability to compete are substantially dependent on our patents, proprietary formulations and trademarks. Although we believe that the patents and associated trademarks and licenses are valid, there can be no assurance that they will not be challenged and subsequently invalidated and/or canceled. The invalidation or cancellation of any one or all of the patents or trademarks would significantly damage our commercial prospects. Further, we may find it necessary to legally challenge parties infringing our patents or trademarks or licensed trademarks to enforce our rights thereto. There can be no assurance that any of the patents would ultimately be held valid or that efforts to defend any of the patents, trade secrets, know-how or other IP rights would be successful.
The patent positions of pharmaceutical and biotechnology companies, such as ours, are uncertain and involve complex legal and factual issues. We own numerous U.S. and foreign patents and a number of pending patent applications that cover various aspects of our drug candidates and technologies. There can be no assurance that patents that have issued will be held valid and enforceable in a court of law. Even for patents that are held valid and enforceable, the legal process associated with obtaining such a judgment is time consuming and costly. Additionally, issued patents can be subject to opposition or other proceedings that can result in the revocation of the patent or maintenance of the patent in amended form (and potentially in a form that renders the patent without commercially relevant and/or broad coverage). Further, our competitors may be able to circumvent and otherwise design around our patents. Even if a patent is issued and enforceable, because development and commercialization of pharmaceutical products can be subject to substantial delays, patents may expire early and provide only a short period of protection, if any, following the commercialization of a product encompassed by our patents. We may have to participate in interference proceedings declared by the USPTO, which could result in a loss of the patent and/or substantial cost to us.
| 27 |
We have filed patent applications, and plan to file additional patent applications, covering various aspects of our drug candidates and technologies. There can be no assurance that the patent applications for which we apply would actually be issued as patents, or do so with commercially relevant and/or broad coverage. The coverage claimed in a patent application can be significantly reduced before the patent is issued. The scope of our claim coverage can be critical to our ability to enter into licensing transactions with third-parties and our right to receive royalties from our collaboration partnerships. Since publication of discoveries in scientific or patent literature often lags behind the date of such discoveries, we cannot be certain that we were the first inventor of inventions covered by our patents or patent applications. In addition, there is no guarantee that we will be the first to file a patent application directed to an invention.
An adverse outcome in any judicial proceeding involving IP, including patents, could subject us to significant liabilities to third-parties, require disputed rights to be licensed from or to third-parties or require us to cease using the technology in dispute. In those instances where we seek an IP license from another, we may not be able to obtain the license on a commercially reasonable basis, if at all, thereby raising concerns on our ability to freely commercialize our technologies and/or products. It is also possible that we or our licensors or licensees will fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Moreover, in some circumstances, we may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, covering technology that we license from or license to third parties and are reliant on our licensors or licensees. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. If our current or future licensors or licensees fail to establish, maintain or protect such patents and other intellectual property rights, such rights may be reduced or eliminated. If our licensors or licensees are not fully cooperative or disagree with us as to the prosecution, maintenance or enforcement of any patent rights, such patent rights could be compromised.
Issued patents covering our product candidates could be found invalid or unenforceable if challenged in court.
If we or one of our licensing partners initiated legal proceedings against a third-party to enforce a patent covering one of our product candidates, the defendant could counterclaim that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Third-parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in revocation or amendment to our patents in such a way that they no longer cover our product candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Such a loss of patent protection would have a material adverse impact on our business.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third-parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our inventions in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
| 28 |
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third-parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
If we infringe on the intellectual property rights of others, our business and profitability may be adversely affected.
Our commercial success will also depend, in part, on us and our collaborative partners not infringing on the patents or proprietary rights of others. There can be no assurance that the technologies and products used or developed by our collaborative partners and marketed and sold by us will not infringe such rights. If such infringement occurs and neither we nor our collaborative partner is able to obtain a license from the relevant third-party, we will not be able to continue the development, manufacture, use, or sale of any such infringing technology or product. There can be no assurance that necessary licenses to third-party technology will be available at all, or on commercially reasonable terms. In some cases, litigation or other proceedings may be necessary to defend against or assert claims of infringement or to determine the scope and validity of the proprietary rights of third-parties. Any potential litigation could result in substantial costs to, and diversion of, our resources and could have a material and adverse impact on us. An adverse outcome in any such litigation or proceeding could subject us to significant liabilities, require us to cease using the subject technology or require us to license the subject technology from the third-party, all of which could have a material adverse effect on our business.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from third-parties or otherwise experience disruptions to our business relationships with our licensors, we could lose license rights that are important to our business.
We are a party to a number of intellectual property license agreements that are important to our business and we expect to enter into additional license agreements in the future. Our existing license agreements impose, and we expect that future license agreements will impose, various diligence, milestone payment, royalty and other obligations on us. If we fail to comply with our obligations under these agreements, or we are subject to a bankruptcy, the licensor may have the right to terminate the license, in which event we would not be able to market products covered by the license.
We may need to obtain licenses from third-parties to advance our research, and we have done so from time to time. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop the affected product candidates, which could harm our business significantly. We cannot provide any assurances that third-party patents do not exist which might be enforced against our current product candidates or future products, resulting in either an injunction prohibiting the sales, or, with respect to the sales, an obligation on our part to pay royalties and/or other forms of compensation to third-parties.
| 29 |
In many cases, patent prosecution of our licensed technology is controlled solely by the licensor. If our licensors fail to obtain and maintain patent or other protection for the proprietary intellectual property we license from them, we could lose our rights to the intellectual property or our exclusivity with respect to those rights, and our competitors could market competing products using the intellectual property. In certain cases, we control the prosecution of patents resulting from licensed technology. In the event we breach any of our obligations related to such prosecution, we may incur significant liability to our licensing partners. Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues and is complicated by the rapid pace of scientific discovery in our industry. Disputes may arise regarding intellectual property subject to a licensing agreement, including:
| · | The scope of rights granted under the license agreement and other interpretation-related issues; | |
| · | The extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; | |
| · | The sublicensing of patent and other rights under our collaborative development relationships; | |
| · | Our diligence obligations under the license agreement and what activities satisfy those diligence obligations; | |
| · | The ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and | |
| · | The priority of invention of patented technology. |
If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid, is unenforceable and/or is not infringed, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
Interference proceedings provoked by third-parties or brought by us may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common stock underlying the units.
| 30 |
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biotechnology industry involve both technological and legal complexity, and is therefore obtaining and enforcing biotechnology patents is costly, time-consuming and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
Patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our and our licensors’ patent applications and the enforcement or defense of our or our licensors’ issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. The USPTO, is currently developing regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, were enacted March 16, 2013. However, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third-parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
We employ individuals who were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee’s former employers or other third-parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could adversely impact our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may also be subject to claims that former employees, collaborators or other third-parties have an ownership interest in our patents or other intellectual property. We may have in the future ownership disputes arising, for example, from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
| 31 |
Our inability to protect our confidential information and trade secrets would harm our business and competitive position.
In addition to seeking patents for some of our technology and products, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts both within and outside the United States may be less willing or unwilling to protect trade secrets. If a competitor lawfully obtained or independently developed any of our trade secrets, we would have no right to prevent such competitor from using that technology or information to compete with us, which could harm our competitive position.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and/or applications will be due to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and/or applications. The USPTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. Non-compliance may result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors might be able to enter the market and this circumstance would have a material adverse effect on our business.
Risks Related to Our Business Operations
We operate in an extremely competitive environment and there can be no assurances that competing technologies would not harm our business development.
We are engaged in a rapidly evolving field. Competition from numerous pharmaceutical companies including for oncology orphans Galena BioPharma; Merck Sharp & Dohme; Immunogen, Inc. Immunomedics, Inc.; Genentech; Macrogenics; Genmab; Incyte Corporation; Eisai Inc. Bayer. ArQule; AstraZeneca; Novartis; Daiichi Sankyo Inc.; GlaxoSmithKline; Advenchen Laboratories LLC, Stemline Therapeutics, Inc., Rexahn Pharmaceuticals, Inc. and Peregrine Pharmaceuticals, Inc., and for protein delivery products Nektar’s PEG technology, Flamel’s Medusa platform offering, a hydrogel depot formulation, Versartis’ XTEN technology which recombinant polypeptide fusion protein, nanoparticle technology from Alkermes, Durect Corp’s long-acting technology, Debiopharm Group’s drug delivery based on polylactic-co-glycolic acid (PLGA),and Halozyme’s ENHANZE drug delivery technology, as well as research and academic institutions, is intense and expected to increase. The large and rapidly growing market for liposomal drugs and oncology treatments is likely to attract new entrants. Numerous biotechnology and pharmaceutical companies are focused on developing new liposomal drug delivery systems and cancer treatments. Many, if not all, of these companies have greater financial and other resources and development capabilities than we do. Many of our competitors also have greater collective experience in undertaking pre-clinical and clinical testing of products, obtaining regulatory approvals and manufacturing and marketing prescription pharmaceutical products. There can be no assurance that our under development drug candidates will be more effective or achieve greater market acceptance than competitive products, or that our competitors will not succeed in developing products and technologies that are more effective than those being developed by us or that would render our products and technologies less competitive or obsolete. Additionally, there can be no assurance that the development by others of new or improved drugs will not make our pharmaceutical products superfluous or obsolete. See “Competition.”
| 32 |
We are a party to collaboration agreements and other significant agreements which contain complex commercial terms that could result in disputes, litigation or indemnification liability that could adversely affect our business, results of operations and financial condition.
We currently derive, and expect to derive in the foreseeable future, all of our revenue from collaboration agreements with biotechnology and pharmaceutical companies. These collaboration agreements contain complex commercial terms, including:
| · | Clinical development and commercialization obligations that are based on certain commercial reasonableness performance standards that can often be difficult to enforce if disputes arise as to adequacy of our partner’s performance; | |
| · | Research and development performance and reimbursement obligations for our personnel and other resources allocated to partnered drug candidate development programs; | |
| · | Clinical and commercial manufacturing agreements, some of which are priced on an actual cost basis for products supplied by us to our partners with complicated cost allocation formulas and methodologies; | |
| · | Intellectual property ownership allocation between us and our partners for improvements and new inventions developed during the course of the collaboration; | |
| · | Royalties on drug sales based on a number of complex variables, including net sales calculations, geography, scope of patent claim coverage, patent life, generic competitors, bundled pricing and other factors; and | |
| · | Indemnity obligations for intellectual property infringement, product liability and certain other claims. |
From time to time, we have informal dispute resolution discussions with third-parties regarding the appropriate interpretation of the complex commercial terms contained in our agreements. One or more disputes may arise or escalate in the future regarding our collaboration agreements, transaction documents, or third-party license agreements that may ultimately result in costly litigation and unfavorable interpretation of contract terms, which would have a material adverse effect on our business, financial condition and results of operations.
Governments may impose price controls, which may adversely affect our future profitability.
We intend to seek approval to market our product candidates in both the United States and in foreign jurisdictions. In some foreign countries and jurisdictions, particularly in the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a drug candidate. To obtain reimbursement or pricing approval in some countries, we may be required to conduct clinical trials to compare the cost effectiveness of our drug candidates to other available therapies, which is time consuming and costly. If reimbursement of our future products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability.
Write-offs related to the impairments of our long-lived assets, including goodwill and indefinite-lived intangible assets, and other non-cash charges such as share-based payments may adversely impact our results of operations.
We may incur significant non-cash charges related to impairments of our long-lived assets, including goodwill and indefinite-lived intangible assets. Although we did not record any such charges during 2015, we are required to perform periodic impairment reviews of those assets at least annually. In 2012, In-Process Research and Development, or IPR&D, acquired from Serum Institute, was immediately impaired as it was not acquired in connection with a business combination. The carrying value of goodwill on our balance sheet that is subject to impairment reviews was approximately $3.28 million at June 30, 2016 and December 31, 2015, and $3.47 million at December 31, 2014 and the carrying value of our indefinite-lived assets was $9.24 million at June 30, 2016 and December 31, 2015, and $9.75 million at December 31, 2014. To the extent future reviews conclude that the expected future cash flows generated from our business activities are not sufficient to recover the carrying value of these assets, we will be required to measure and record an impairment charge to write-down these assets to their realizable values and those impairment charges could be equal to the entire carrying value.
| 33 |
We completed our last review during the fourth quarter of 2015 and determined that goodwill and indefinite-lived intangible assets were not impaired as of December 31, 2015. However, there can be no assurance that upon completion of subsequent reviews a material impairment charge will not be recorded. If future periodic reviews determine that our assets are impaired and a write-down is required, it will adversely impact our operating results.
In addition, we recorded non-cash charges of approximately $2.0 million for share-based payments during the six months ended June 30, 2016, and $2.59 million and $1.51 million for share-based payments during 2015 and 2014, respectively. In the future, this amount could fluctuate materially as the Company expects to continue to issue share-based payments awards.
Potential new accounting pronouncements or legislative actions may adversely impact our future financial position or results of operations.
Future changes in financial accounting standards may cause adverse, unexpected fluctuations in the timing of the recognition of revenues or expenses, and may affect our financial position or results of operations. New pronouncements may occur in the future and may cause us to be required to make changes in our accounting policies in the future. Compliance with changing regulation of corporate governance and public disclosure may result in additional expenses. Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, new SEC regulations, Public Company Accounting Oversight Board, or PCAOB, pronouncements and NASDAQ rules, are creating uncertainty for companies such as ours and insurance, accounting and auditing costs are high as a result of this uncertainty and other factors.
We are committed to maintaining high standards of corporate governance and public disclosure. As a result, we intend to invest all reasonably necessary resources to comply with evolving standards, and this investment may result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities.
Varying interpretations of existing pronouncements and rules have occurred with frequency.
Varying interpretations of existing pronouncements of accounting policies or accounting treatments of existing transactions may cause us to have to restate previously reported result of operations.
For example, in January 2014 we completed the Acquisition that we determined to be a reverse merger business combination. We allocated the purchase price consideration to the assets acquired and liabilities assumed at their estimated fair values as of the date of acquisition. Our determination that the Acquisition meets the criteria for a business combination was based on our best knowledge of the facts and circumstances surrounding the transaction, and requires the application of our judgment. Changes to this determination will result in the transaction to be accounted for as a recapitalization, with no goodwill recorded, which could cause a material change in our reported results of operations and could cause the Company to have to amend prior periodic or other filings with the SEC, at further expense to the Company.
In addition, we do not consider the Company to be a development stage entity for financial reporting presentation purposes. A determination that the Company is a development stage entity could cause a material change in our reported results of operations and could cause the Company to have to amend prior periodic or other filings with the SEC, at further expense to the Company.
| 34 |
Our future success depends on our ability to retain key employees, consultants and advisors and to attract, retain and motivate qualified personnel.
We are highly dependent on principal members of our executive team and key employees, the loss of whose services may adversely impact the achievement of our objectives. Recruiting and retaining other qualified employees, consultants and advisors for our business, including scientific and technical personnel, will also be critical to our success. There is currently a shortage of skilled executives in our industry, which is likely to continue. As a result, competition for skilled personnel is intense and the turnover rate can be high. We may not be able to attract and retain personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for individuals with similar skill sets. In addition, failure to succeed in preclinical or clinical studies may make it more challenging to recruit and retain qualified personnel. The inability to recruit or loss of the services of any executive, key employee, consultant or advisor may impede the progress of our research and development objectives.
We will need to expand our organization and we may experience difficulties in managing this growth, which could disrupt our operations.
As of June 30, 2016, we had six full-time employees. As we mature, we may need to expand our full-time employee base and to hire more consultants and contractors. Our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Any future growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional product candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and/or grow revenues could be reduced, and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize product candidates and compete effectively will depend, in part, on our ability to effectively manage any future growth.
Our employees, principal investigators, consultants and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, principal investigators, consultants and commercial partners. Misconduct by these parties could include intentional failures to comply with the regulations of the FDA and non-U.S. regulators, provide accurate information to the FDA and non-U.S. regulators, comply with healthcare fraud and abuse laws and regulations in the United States and abroad, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and cause serious harm to our reputation or could cause regulatory agencies not to approve our product candidates. While we intend to adopt a comprehensive code of conduct applicable to all of our employees, it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
| 35 |
We face potential product liability, and, if successful claims are brought against us, we may incur substantial liability and costs. If the use of our product candidates harms patients, or is perceived to harm patients even when such harm is unrelated to our product candidates, our regulatory approvals could be revoked or otherwise negatively impacted and we could be subject to costly and damaging product liability claims.
The use of our product candidates in clinical studies and the sale of any products for which we obtain marketing approval exposes us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our products. There is a risk that our product candidates may induce adverse events. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| · | Impairment of our business reputation; | |
| · | Withdrawal of clinical study participants; | |
| · | Costs due to related litigation; | |
| · | Distraction of management’s attention from our primary business; | |
| · | Substantial monetary awards to patients or other claimants; | |
| · | The inability to commercialize our product candidates; and | |
| · | Decreased demand for our product candidates, if approved for commercial sale. |
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third-parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials or other work-related injuries, this insurance may not provide adequate coverage against potential liabilities. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
We incur significant costs as a result of operating as a public company, and our management devotes substantial time to public company compliance initiatives.
As a U.S. public company, we have incurred and will continue to incur significant legal, accounting and other expenses that we did not incur as a U.K. public company. In addition, the Sarbanes-Oxley Act, as well as rules subsequently implemented by the SEC and the NASDAQ Capital Market, has imposed various requirements on public companies. In July 2010, the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, was enacted. There are significant corporate governance and executive compensation related provisions in the Dodd-Frank Act that require the SEC to adopt additional rules and regulations in these areas such as “say on pay” and proxy access. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain our current levels of such coverage.
| 36 |
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
We are subject to the periodic reporting requirements of the Exchange Act of 1934, as amended, or the Exchange Act. We designed our disclosure controls and procedures to reasonably assure that information we must disclose in reports we file or submit under the Exchange Act is accumulated and communicated to management, and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected.
Risks Related to Investment in Our Securities
There is no public market for the Series B Preferred Stock or the warrants to purchase shares of our common stock being offered by us in this offering.
There is no established public trading market for the Series B Preferred Stock or the warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the Series B Preferred Stock or the warrants on any national securities exchange or other nationally recognized trading system, including The NASDAQ Capital Market. Without an active market, the liquidity of the Series B Preferred Stock and the warrants will be limited.
An active, liquid and orderly market for our common stock may not develop, and you may not be able to sell common stock underlying the units at or above the public offering price.
Prior to this offering, our common stock is traded on the OTCQB quotation system, which is a FINRA-sponsored entity and operated inter-dealer automated quotation system for equity securities not included in a national exchange. Quotation of our securities on the OTCQB limits the liquidity and price of our common stock more than if our common stock were quoted or listed on the NYSE or the NASDAQ, which are national securities exchanges. Although we have applied to list our common stock on the NASDAQ Capital Market, an active trading market for our common stock may never develop or be sustained following this offering. We and the underwriter will determine the initial public offering price of our Series B Preferred Stock stock through negotiation. This price will not necessarily reflect the price at which investors in the market will be willing to buy and sell the common stock underlying the Series B Preferred Stock and warrants following this offering. In addition, an active trading market for our common stock may not develop following the consummation of this offering or, if it is developed, may not be sustained. The lack of an active market may impair your ability to sell common stock issuable upon conversion of the Series B Preferred Stock and exercise of the warrants offered by this prospectus at the time you wish to sell them or at a price that you consider reasonable. An inactive market may also impair our ability to raise capital by selling common stock and may impair our ability to acquire other businesses, applications or technologies using our common stock as consideration, which, in turn, could materially adversely affect our business.
| 37 |
Our common stock is a “penny stock” under SEC rules. It may be more difficult to sell securities classified as “penny stock.”
Our common stock is a “penny stock” under applicable SEC rules (generally defined as non-exchange traded stock with a per-share price below $5.00). Unless we maintain a per-share price above $5.00, these rules impose additional sales practice requirements on broker-dealers that recommend the purchase or sale of penny stocks to persons other than those who qualify as “established customers” or “accredited investors.” For example, broker-dealers must determine the appropriateness for non-qualifying persons of investments in penny stocks. Broker-dealers must also provide, prior to a transaction in a penny stock not otherwise exempt from the rules, a standardized risk disclosure document that provides information about penny stocks and the risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, disclose the compensation of the broker-dealer and its salesperson in the transaction, furnish monthly account statements showing the market value of each penny stock held in the customer’s account, provide a special written determination that the penny stock is a suitable investment for the purchaser, and receive the purchaser’s written agreement to the transaction. Legal remedies available to an investor in “penny stocks” may include the following:
If a “penny stock” is sold to the investor in violation of the requirements listed above, or other federal or states securities laws, the investor may be able to cancel the purchase and receive a refund of the investment.
If a “penny stock” is sold to the investor in a fraudulent manner, the investor may be able to sue the persons and firms that committed the fraud for damages.
However, investors who have signed arbitration agreements may have to pursue their claims through arbitration.
These requirements may have the effect of reducing the level of trading activity, if any, in the secondary market for a security that becomes subject to the penny stock rules. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers from effecting transactions in our securities, which could severely limit the market price and liquidity of our securities. These requirements may restrict the ability of broker-dealers to sell our common stock and may affect your ability to resell common stock underlying the units.
Many brokerage firms will discourage or refrain from recommending investments in penny stocks. Most institutional investors will not invest in penny stocks. In addition, many individual investors will not invest in penny stocks due, among other reasons, to the increased financial risk generally associated with these investments. For these reasons, penny stocks may have a limited market and, consequently, limited liquidity. We can give no assurance that our common stock will not be classified as a “penny stock” in the future.
We are applying for listing of our common stock on the NASDAQ Capital Market. We can provide no assurance that our common stock qualify to be listed, and if listed, that our common stock will continue to meet NASDAQ listing requirements. If we fail to comply with the continuing listing standards of the NASDAQ Capital Market, our securities could be delisted.
We expect that our common stock will be eligible to be listed on the NASDAQ Capital Market. However, we can provide no assurance that our application will be approved, and that an active trading market for our common stock will develop and continue. If, after listing, we fail to satisfy the continued listing requirements of the NASDAQ Capital Market, such as the corporate governance requirements or the minimum closing bid price requirement, NASDAQ may take steps to delist our common stock. Such a delisting would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase common stock underlying the units when you wish to do so. In the event of a delisting, we can provide no assurance that any action taken by us to restore compliance with listing requirements would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the NASDAQ minimum bid price requirement or prevent future non-compliance with NASDAQ's listing requirements.
| 38 |
The market price of our stock may be highly volatile, and you may not be able to sell shares of stock underlying the units at or above the price of the units.
Companies trading in the stock market in general have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our stock, regardless of our actual operating performance.
The market price of our stock may be volatile. Our stock price could be subject to wide fluctuations in response to a variety of factors, including the following:
| · | Adverse results or delays in pre-clinical or clinical studies; | |
| · | Inability to obtain additional funding; | |
| · | Any delay in filing an IND or BLA for any of our product candidates and any adverse development or perceived adverse development with respect to the FDA’s review of that IND or BLA; | |
| · | Failure to develop successfully our product candidates; | |
| · | Failure to maintain our existing strategic collaborations or enter into new collaborations; | |
| · | Failure by us or our licensors and strategic collaboration partners to prosecute, maintain or enforce our intellectual property rights; | |
| · | Changes in laws or regulations applicable to future products; | |
| · | Inability to obtain adequate product supply for our product candidates or the inability to do so at acceptable prices; | |
| · | Adverse regulatory decisions; | |
| · | Introduction of new products, services or technologies by our competitors; | |
| · | Failure to meet or exceed financial projections we may provide to the public; | |
| · | Failure to meet or exceed the financial projections of the investment community; | |
| · | The perception of the pharmaceutical industry by the public, legislatures, regulators and the investment community; | |
| · | Announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us, our strategic collaboration partner or our competitors; | |
| · | Disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; | |
| · | Additions or departures of key scientific or management personnel; | |
| · | Significant lawsuits, including patent or stockholder litigation; | |
| · | Changes in the market valuations of similar companies; | |
| · | Sales of our common stock by us or our stockholders in the future; and | |
| · | Trading volume of our common stock. |
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
Our executive officers, directors, and their affiliates and other principal stockholders beneficially own approximately 83% of our outstanding common stock as of the date of this prospectus. Therefore, these stockholders will have the ability to influence us through their ownership positions. Further, our largest shareholder, Pharmsynthez, has beneficial ownership of 5,378,088 shares of common stock, on an as-converted basis. These shares represent beneficial ownership of approximately 52% of our common stock as of the date of this prospectus. These stockholders may be able to determine all matters requiring stockholder approval. For example, these stockholders, acting together, may be able to control elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may believe are in your best interest as one of our stockholders.
| 39 |
We have entered into several agreements with our major stockholders.
These arrangements may not have been negotiated at arm’s length and may contain terms and conditions that are not in our best interest and would not otherwise be applicable if we entered into arrangements with a third-party not affiliated with us. Although we did, and will, attempt to negotiate agreements at arm’s length, some of the agreement parties may be considered affiliates of ours, which may result in conflicts of interest. See the section entitled “Certain Relationships and Related Party Transactions” below.
We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
We could be an emerging growth company for up to approximately five years from our initial public offering, which was declared effective March 22, 2012, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700.0 million as of any June 30 before that time or if we have total annual gross revenue of $1.0 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31 or, if we issue more than $1.0 billion in nonconvertible debt during any three-year period before that time, we would cease to be an emerging growth company immediately. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company” which would allow us to take advantage of many of the same exemptions from disclosure requirements, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. In the preparation of our accounting reports, we have generally taken the position not to avail ourselves of this exemption from new or revised accounting standards and, therefore, have continued to be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because pharmaceutical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
We do not intend to pay dividends on our common stock or preferred stock so any returns will be limited to the value of our stock.
We have never declared or paid any cash dividends on our common stock or preferred stock. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any return to common or preferred stockholders will therefore be limited to the appreciation of their stock.
| 40 |
Risks Related to this Offering
Series B Preferred Stock is an equity security and is junior in right of payment to any future indebtedness.
The shares of Series B Preferred Stock are equity interests in the Company and do not constitute indebtedness. The shares of Series B Preferred Stock will rank junior in right of payment to any future indebtedness, and equal in right of payment with all of our outstanding preferred stock, and the terms of the Series B Preferred Stock will not prohibit us from issuing additional securities in the future that rank senior (other than senior stock as described below) or equal in right of payment with the Series B Preferred Stock. Any future indebtedness may also restrict payment of dividends on the Series B Preferred Stock.
Series B Preferred Stock is junior in rights and preferences to our Series A Preferred Stock.
The terms of the Series A Preferred Stock are expressly senior to Series B Preferred Stock. The terms of Series A Preferred Stock may restrict dividend payments on Series B Preferred Stock unless full dividends for all of our outstanding Series A Preferred Stock have been declared and paid or set aside for payment for the relevant period or periods specified by the terms of such preferred stock. This could adversely affect your rights as a holder of Series B Preferred Stock.
Series B Preferred Stock may be junior in rights and preferences to our future preferred stock.
We may issue preferred stock in the future, the terms of which are expressly senior to Series B Preferred Stock. The terms of any such future preferred stock may restrict dividend payments on Series B Preferred Stock unless full dividends for all of our outstanding preferred stock senior to Series B Preferred Stock have been declared and paid or set aside for payment for the relevant period or periods specified by the terms of such preferred stock. If we issue such shares of preferred stock in the future, the rights of holders of Series B Preferred Stock or the market price of the Preferred Stock could be adversely affected.
Dividends on Series B Preferred Stock are non-cumulative.
Dividends on Series B Preferred Stock are non-cumulative and are payable when, as and if declared by our board of directors. Consequently, if our board of directors does not authorize and declare a dividend for any dividend period prior to the related dividend payment date, holders of Series B Preferred Stock would not be entitled to receive a dividend for that dividend period, and the undeclared dividend will not accrue and not be payable, whether or not dividends are declared for any subsequent dividend period with respect to Series B Preferred Stock. We will have no obligation to pay dividends accrued for a dividend period after the dividend payment date for that period if our board of directors has not declared a dividend prior to such dividend payment date, whether or not dividends are declared for any subsequent period with respect to Series B Preferred Stock.
Our ability to declare dividends on Series B Preferred Stock may be limited.
Unlike indebtedness, where principal and interest customarily are payable on specified due dates, in the case of Series B Preferred Stock, (i) dividends are payable only if and when declared by our board of directors out of funds legally available for such payments; (ii) as a corporation, we are subject to restrictions on dividend payments and redemption payments out of lawfully available funds; and (iii) there are no restrictions on our business or operations or on our ability to incur indebtedness, issue additional preferred stock or engage in any transactions. Our ability to pay dividends on Series B Preferred Stock is also subject to restrictions in our Series A Preferred Stock, which ranks equally with Series B Preferred Stock in right of payment.
| 41 |
Holders of Series B Preferred Stock will have limited voting rights.
Holders of Series B Preferred Stock have no rights with respect to matters that generally require the approval of holders of our Common Stock. Holders of Series B Preferred Stock will have voting rights only as required by law, in articles of incorporation, and in the certificate of designations for Series B Preferred Stock. See “Description of the Series B Preferred Stock—Voting Rights.”
If investors are able to resell their Series B Preferred Stock, many other factors may affect the price they receive, which may be lower than they believe to be appropriate.
If investors are able to resell their shares of Series B Preferred Stock, the price they receive will depend on many other factors that may vary over time, including:
| · | the number of potential buyers; |
| · | the level of liquidity of Series B Preferred Stock; |
| · | our financial performance; |
| · | the amount of indebtedness we have outstanding; |
| · | the level, direction and volatility of market interest rates generally; |
| · | the market for similar securities; |
| · | the market price of our Common Stock; and |
| · | the terms of Series B Preferred Stock. |
As a result of these factors, investors may only be able to sell their Series B Preferred Stock at prices that may be below those they believe to be appropriate, including prices below the price they paid for Series B Preferred Stock.
A significant portion of our total outstanding shares of common stock are restricted from immediate resale but may be sold into the market in the near future, which could cause our stock price to decline.
A significant number of our outstanding shares are subject to contractual lock-up restrictions on resale that extend for ninety (90) days after the date of this prospectus pursuant to lock-up agreements that our officers, directors and certain stockholders have signed, as more fully described in the section entitled “Underwriting” in this prospectus. If these stockholders sell, or indicate an intent to sell, substantial amounts of our common stock in the public market after the expiration of the applicable lock-up period, the trading price of our common stock could decline significantly and could decline below the public offering price.
After certain of the lock-up agreements pertaining to this offering expire, an approximate 6.9 million additional shares will be eligible for sale in the public market 90 days following this offering. Furthermore, 671,853 shares subject to outstanding options and 681,878 shares reserved for future issuance, under our 2014 Plan, will become eligible for sale in the public market in the future, subject to certain legal and contractual limitations. If our existing stockholders sell substantial amounts of our common stock in the public market, or if the public perceives that such sales could occur, this could have an adverse impact on the market price of our common stock, even if there is no relationship between such sales and the performance of our business.
| 42 |
We will have broad discretion in how we use the net proceeds from this offering. We may not use these proceeds effectively, which could affect our results of operations and cause our stock price to decline.
We will have considerable discretion in the application of the net proceeds that we receive from this offering. We intend to use the net proceeds from this offering to commercialize Virexxa and to fund development and clinical trials of OncoHist and ErepoXen, as well as for working capital and other general corporate purposes. As a result, investors will be relying on management’s judgment with only limited information about our specific intentions for the use of the balance of the net proceeds of this offering. We may use the net proceeds for purposes that do not yield a significant return or any return at all for our stockholders. In addition, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
Investors in this offering will experience immediate and substantial dilution in the book value per share of common stock as a result of this offering.
Investors in this offering will experience immediate dilution in their net tangible book value per share to the extent of the difference between the conversion price per share of common stock and the "adjusted" net tangible book value per share after giving effect to the offering. After giving effect to the sale by us of 2,500,000 units offered in this offering, and after deducting the underwriters discounts and commissions and other estimated offering expenses payable by us, investors in this offering can expect an immediate dilution of $3.40 per share on the common stock underlying the units, assuming no exercise of the warrants. In addition, in the past, we issued options and warrants to acquire shares of common stock at prices significantly below the public offering price. To the extent these options are ultimately exercised, you will sustain future dilution on common stock underlying the units. We may also acquire or license other technologies or finance strategic alliances by issuing equity, which may result in additional dilution to our stockholders. See the section entitled "Dilution" below.
| 43 |
Special Note Regarding Forward-Looking Statements
This prospectus contains forward-looking statements that are based on our management’s belief and assumptions and on information currently available to our management. Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to future events or our future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Forward-looking statements in this prospectus include, but are not limited to, statements about: our plans to continue the development of our proposed drug candidates; our expectations regarding the nature, timing and extent of clinical trials and proposed clinical trials; our expectations regarding the timing for proposed submissions of regulatory filings, including but not limited to any IND filing or any new drug application; the nature, timing and extent of collaboration arrangements; the expected results pursuant to collaboration arrangements including the receipts of future payments that may arise pursuant to collaboration arrangements; the outcome of our plans to obtain regulatory approval of our drug candidates; the outcome of our plans for the commercialization of our drug candidates; our plans to address certain markets, engage third-party manufacturers, and evaluate additional drug candidates for subsequent commercial development, and the likelihood and extent of competition to our drug candidates.
In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under “Risk Factors” and elsewhere in this prospectus. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those implied or projected by the forward-looking statements. No forward-looking statement is a guarantee of future performance. You should read this prospectus and the documents that we reference in this prospectus and have filed with the SEC as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from any future results expressed or implied by these forward-looking statements.
The forward-looking statements in this prospectus represent our views as of the date of this prospectus. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should therefore not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this prospectus.
The single most pressing factor that could cause actual results to differ materially and adversely is our need to raise additional working capital for the purpose of further developing our various drug candidates. We estimate that we have less than six months of working capital as of June 30, 2016 and our future results could differ materially and adversely if we are unable to raise additional working capital. Other factors that could cause actual results to differ materially include without limitation:
| · | We have a limited operating history, have incurred significant operating losses since our inception and expect to incur significant losses for the foreseeable future. We may never generate any revenue or become profitable or, if we achieve profitability, we may not be able to sustain it. | |
| · | Our business currently depends substantially on the success of Virexxa and ErepoXen, which will require significant clinical testing before we can seek regulatory approval and potentially launch commercial sales. If we are unable to obtain regulatory approval for, or successfully commercialize, Virexxa and ErepoXen, our business will be materially harmed. | |
| · | Any termination or suspension of, or delays in the commencement or completion of, our planned clinical trials could result in increased costs to us, delay or limit our ability to generate revenue and adversely affect our commercial prospects. | |
| · | All of our product candidates are still in preclinical or early-stage clinical development. If we are unable to commercialize our product candidates or if we experience significant delays in obtaining regulatory approval for, or commercializing, any or all of our product candidates, our business will be materially and adversely affected. | |
| · | We rely on third-parties to conduct some or all aspects of our product manufacturing, research and preclinical and clinical testing, and these third-parties may not perform satisfactorily. | |
| · | Our rights to develop and commercialize our product candidates are subject in part to the terms and conditions of licenses granted to us by other companies. | |
| · | Our success depends on our ability to protect our intellectual property and our proprietary technologies. |
| 44 |
Use of proceeds
Based on an assumed public offering price of $4.00 per unit, the minimum price for initial listing on the NASDAQ Capital Market, we estimate that the net proceeds to us from the sale of the units that we are offering will be approximately $9.55 million after deducting underwriting discounts and commissions and estimated offering expenses (assuming none of the warrants issued in this offering are exercised). In addition, if all of the warrants offered pursuant to this prospectus are exercised in full for cash, we will receive approximately an additional $ in cash.
A $1.00 increase (decrease) in the assumed public offering price of $4.00 per unit, the minimum price for initial listing on the NASDAQ Capital Market, would increase (decrease) the net proceeds from this offering by approximately $2.5 million, assuming the number of units offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, after giving effect to the sale of 2,500,000 units at the assumed offering price of $4.00. Similarly, each increase (decrease) of one million units offered by us would increase (decrease) the net proceeds from this offering by approximately $4.0 million, assuming the assumed public offering price remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, after giving effect to the sale of 2,500,000 units at the assumed offering price of $4.00.
The principal purpose of this offering is to increase our financial flexibility. We currently expect to use the net proceeds from this offering as follows:
| · | approximately $3.0 million to fund the ongoing clinical trials; | |
| · | approximately$1.8 million to fund active and planned preclinical programs as well as in-house R&D costs; | |
| approximately $0.5 million to repay outstanding convertible promissory notes with a maturity date of September 1, 2017 and an interest rate of 10% per annum held by Pharmsynthez; and | ||
| · | the remainder to fund general corporate costs. |
Due to the uncertainties inherent in the clinical development and regulatory approval process, it is difficult to estimate with certainty the exact amounts of the net proceeds from this offering that may be used for the above purposes. We may also find it necessary or advisable to use the net proceeds from this offering for other purposes. Accordingly, our management will retain broad discretion over the use of the net proceeds from this offering. The amounts and timing of our expenditures will depend upon numerous factors. For instance, the amounts and timing of our expenditures will in part depend on the time and cost necessary to conduct our Phase II clinical trial for Virexxa, which will largely depend on the number of patient cohorts that we expand as a result of patient responses. Because we cannot predict which cohorts, if any, we will expand, there can be no assurance that our existing capital resources and the net proceeds from this offering will be sufficient to fund our clinical trial for any specific cohort to completion, and we do not expect such amounts to be sufficient to fund the full clinical trial to completion, regulatory approval process and commercialization. Furthermore, the amounts and timing of our expenditures will depend on (1) the time and cost associated with clinical trials and preclinical development of other product programs; (2) the results of any clinical trials and other studies; and (3) other factors described under the heading "Risk Factors" included elsewhere in this prospectus.
Following this offering, we will require substantial capital in order to complete clinical development and commercialize Virexxa and complete the clinical development of any additional product candidates. For additional information regarding our potential capital requirements, see “Even if this offering is successful, we will require substantial additional funding to achieve our goals. Failure to obtain this necessary capital when needed on acceptable terms, or at all, may force us to delay, limit or terminate our product development efforts, other operations or commercialization efforts as described under the heading "Risk Factors.”
Pending these uses, we intend to invest the net proceeds in investment grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government, or hold as cash.
| 45 |
Dividend policy
We have never declared or paid any dividends on our common stock or Series B Preferred Stock. We currently intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business and we do not anticipate paying any cash dividends on our common stock or Series B Preferred Stock in the foreseeable future. Any future determination to pay dividends will be made at the discretion of our board of directors. In addition, any future indebtedness that we may incur could preclude us from paying dividends. Investors should not purchase our common stock or Series B Preferred Stock with the expectation of receiving cash dividends. Our certificate of designation of preferences, rights and limitations of Series A convertible preferred stock provides for a non-cumulative 5% per annum cash dividend on our outstanding Series A Preferred Stock when and if declared by the Board of Directors.
There are no restrictions in our articles of incorporation or bylaws that prevent us from declaring dividends. The Nevada Revised Statutes, however, do prohibit us from declaring dividends where after giving effect to the distribution of the dividend:
| · | We would not be able to pay our debts as they become due in the usual course of business; or | |
| · | Our total assets would be less than the sum of our total liabilities plus the amount that would be needed to satisfy the rights of shareholders who have preferential rights superior to those receiving the distribution. |
| 46 |
Market Price of Our Common Stock AND OTHER RELATED STOCKHOLDER MATTERS
Market Information
There is no established public trading market for the Series B Preferred Stock, or the warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the Series B Preferred Stock or the warrants on any national securities exchange or other nationally recognized trading system, including The NASDAQ Capital Market. Without an active market, the liquidity of the Series B Preferred Stock, and the warrants will be limited.
Our common stock is quoted under the symbol “XBIO” on the OTCQB operated by the OTC Markets Group, Inc. The criteria for listing on the OTCQB include that we remain current in our SEC reporting. Our reporting is presently current, and since inception, we have filed our SEC reports on time. Prior to entering into the Scheme of Agreement under Part 26 of the Companies Act 2006 of England and Wales pursuant on January 2014, the stock of the accounting acquirer, Xenetic Biosciences PLC, was traded on the London AIM stock exchange. We have applied to list our common stock on the Nasdaq Capital Market.
Only a limited market exists for our common stock. There is no assurance that a regular trading market will develop, or if developed, that it will be sustained. Without an active market, the liquidity of the common stock will be limited. Therefore, a stockholder may be unable to sell the underlying shares of our common stock.
The following table sets forth the range of high and low prices for our common stock for each of the periods indicated as reported by the OTCQB. These quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
| Year Ending December 31, 2014 | High Price | Low Price | ||||||
| 1st Quarter Ended March 31, 2014 | $ | 330.03 | $ | 23.10 | ||||
| 2nd Quarter Ended June 30, 2014 | $ | 35.97 | $ | 9.90 | ||||
| 3rd Quarter Ended September 30, 2014 | $ | 32.67 | $ | 13.86 | ||||
| 4th Quarter Ended December 31, 2014 | $ | 22.44 | $ | 5.78 | ||||
| Year Ending December 31, 2015 | ||||||||
| 1st Quarter Ended March 31, 2015 | $ | 8.58 | $ | 5.94 | ||||
| 2nd Quarter Ended June 30, 2015 | $ | 8.25 | $ | 5.94 | ||||
| 3rd Quarter Ended September 30, 2015 | $ | 22.11 | $ | 6.27 | ||||
| 4th Quarter Ended December 31, 2015 | $ | 37.62 | $ | 9.57 | ||||
| Quarterly Periods 2016 | ||||||||
| 1st Quarter Ended March 31, 2016 | $ | 17.00 | $ | 6.80 | ||||
| 2nd Quarter Ended June 30, 2016 | $ | 10.23 | $ | 5.00 | ||||
| 3rd Quarter Ended September 30, 2016 | $ | 5.35 | $ | 3.30 | ||||
| 4th Quarter Ended December 31, 2016 (through October 20, 2016) | $ | 4.75 | $ | 4.00 | ||||
On October 20, 2016, the last reported sales price per share of our common stock was $4.40 and as of October 20, 2016, there were approximately 431 holders of record of our common stock.
| 47 |
Penny Stock
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a market price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on the NASDAQ system, provided that the current price and volume information with respect to transactions in such securities is provided by the exchange or system. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock, to deliver a standardized risk disclosure document prepared by the SEC, that: (a) contains a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading; (b) contains a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to a violation of such duties or other requirements of the securities laws; (c) contains a brief, clear, narrative description of a dealer market, including bid and ask prices for penny stocks and the significance of the spread between the bid and ask price; (d) contains a toll-free telephone number for inquiries on disciplinary actions; (e) defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and (f) contains such other information and is in such form, including language, type size and format, as the SEC shall require by rule or regulation.
The broker-dealer also must provide, prior to effecting any transaction in a penny stock, the customer with (a) bid and offer quotations for the penny stock; (b) the compensation of the broker-dealer and its salesperson in the transaction; and (c) the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and (d) a monthly account statement showing the market value of each penny stock held in the customer’s account.
In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written acknowledgment of the receipt of a risk disclosure statement, a written agreement as to transactions involving penny stocks, and a signed and dated copy of a written suitability statement.
These disclosure requirements may have the effect of reducing the trading activity for our common stock. Therefore, stockholders may have difficulty selling our securities.
Securities Authorized for Issuance under Equity Compensation Plans
We grant stock options and other equity incentive awards pursuant to our Xenetic Biosciences, Inc. Equity Incentive Plan (Plan), which has been approved by our stockholders. The following table sets forth certain information as of June 30, 2016, with respect to the Plan:
| Equity Compensation Plan Information | |||
| Plan Category | Number of Securities to be Issued Upon Exercise of the Outstanding Options, Warrant and Rights | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Referenced in Column (a)) |
| (a) | (b) | (c) | |
| Equity Compensation Plans Approved by Security Holders | 671,853 | $14.99 | 681,878 |
| Equity Compensation Plans Not Approved by Security Holders | 0 |
0 | 0 |
| Total: | 671,853 |
$14.99 | 681,878 |
| 48 |
Capitalization
The following table sets forth our cash, cash equivalents and capitalization as of June 30, 2016:
| ● | on an actual basis; and | |
| ● |
on a pro forma basis to give effect to our sale in this offering of 2,500,000 units at an assumed public offering price of $4.00 per unit, the minimum price for initial listing on the NASDAQ Capital Market, after deducting the underwriting discounts and commissions and estimated offering expenses. |
You should read the following table together with “Description of Capital Stock” appearing elsewhere in this prospectus, and our financial statements and related notes and the information set forth under the headings “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
| As of June 30, 2016 | ||||||||
| Unaudited | Pro Forma (1)(2)(3) | |||||||
| Current Assets | 478,495 | 10,028,495 | ||||||
| Current Liabilities | 3,800,506 | 3,800,506 | ||||||
| Stockholders’ equity: | ||||||||
| Preferred stock, par value $0.001 per share; 10,000,000 shares authorized; no shares issued and outstanding, actual; 970,000 shares of Series A Preferred issued and outstanding, pro forma | – | 970 | ||||||
| Preferred stock, par value $0.001 per share; 10,000,000 shares authorized; no shares issued and outstanding, actual and pro forma | – | – | ||||||
| Common Stock, $0.001 par value; 45,454,546 shares authorized; 9,348,757 shares issued, actual; 10,878,757 shares issued, pro forma; 9,024,872 shares outstanding, actual; 10,554,872 shares outstanding, pro forma | 9,348 | 10,878 | ||||||
| Additional paid-in capital | 150,905,035 | 160,452,535 | ||||||
| Accumulated deficit | (139,474,696 | ) | (139,474,696 | ) | ||||
| Total stockholders’ equity | 6,412,241 | 15,962,241 | ||||||
| Total capitalization | 13,160,711 | 22,710,711 | ||||||
_________________
| (1) | The pro forma gives effect to the sale in this offering of 2,500,000 units at an assumed public offering price of $4.00 per unit, the minimum price for initial listing on the NASDAQ Capital Market, after deducting the underwriting discounts and commissions and estimated offering expenses, and the exchange by SynBio of 970,000 shares of our common stock for 970,000 shares of our Series A Preferred Stock. A $1.00 increase (decrease) in the assumed public offering price of $4.00 per unit would increase (decrease) the amount of current assets, additional paid-in capital, total stockholders’ equity and total capitalization on a pro forma basis by approximately $2.5 million, assuming the number of units offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of one million units offered by us would increase (decrease) current assets, total stockholders’ equity and total capitalization on a pro forma basis by approximately $4.0 million, assuming the assumed public offering price remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will be adjusted based on the actual offering price and other terms of this offering determined at pricing. |
| (2) | Pro-forma Additional Paid in Capital includes approximately $8.5 million estimated fair value, using the Black-Scholes pricing model, for the expected issuance of an aggregate of 2,500,000 Class A Warrants and Class B Warrants in connection with the offering. |
| (3) | Assumes the conversion of all the shares of Series B preferred stock sold in the offering. |
The actual and pro forma information set forth in the table excludes:
| ● | 671,853 shares of common stock issuable upon the exercise of stock options outstanding as of June 30, 2016 at a weighted-average exercise price of $14.99 per share; | |
| ● | 970,000 shares of common stock issuable upon the conversion of Series A Preferred Stock; | |
| ● | 758,347 shares of common stock issuable upon the exercise of outstanding warrants at a weighted-average exercise price of $18.21 per share; and |
| ● | 681,878 shares of common stock reserved for future issuance as of June 30, 2016 under the 2014 Plan. |
| 49 |
Dilution
If you invest in units offered by us in this offering, your interest will be diluted to the extent of the difference between the public offering price per unit and the pro forma net tangible book value per share of our common stock immediately after this offering assuming no value is attributable to the Series A warrants and Series B warrants included in the units sold in this offering. Such calculation does not reflect any dilution associated with the sale and exercise of the Series A warrants or Series B warrants issued as part of the units, which would cause the actual dilution to our public securityholders to be higher, particularly where a cashless exercise is utilized.
The net tangible book value of our common stock as of June 30, 2016 was $(3,195,748), or $(0.35) per share of common stock. Net tangible book value per share represents our total tangible assets less our total tangible liabilities, divided by the number of shares of common stock outstanding on such date.
Net tangible book value dilution per share of common stock underlying the Series B Preferred Stock in each unit to new investors represents the difference between the amount per share of common stock underlying the Series B Preferred Stock in each unit paid by purchasers of common stock in this offering and the pro forma net tangible book value per share of our common stock immediately after the completion of this offering. After giving effect to our sale of 2,500,000 units in this offering at an assumed public offering price of $4.00 per unit, the minimum price for initial listing on the NASDAQ Capital Market, and after deducting underwriting discounts and commissions and estimated offering expenses, our pro forma net tangible book value as of June 30, 2016, would have been $(0.35) per share. This represents an immediate adjusted net tangible book value per share after this offering of $0.60 per share of common stock to existing stockholders and an immediate dilution in net tangible book value of $3.40 per share to purchasers of units in this offering, as illustrated in the following table:
| As of June 30, 2016 | ||||||||
| Actual | Pro forma | |||||||
Assumed public offering price per unit |
$ | 4.00 | ||||||
| Historical net tangible book value per share | $ | (0.35 | ) | |||||
| Increase in net tangible book value per share attributable to investors participating in this offering | $ | 0.96 | ||||||
| Pro forma net tangible book value per share after this offering | $ | 0.60 | ||||||
| Pro forma dilution in net tangible book value per share to investors participating in this offering | $ | 3.40 | ||||||
The above discussion and tables are based on 9,024,872 shares of common stock issued and outstanding as of June 30, 2016, and excludes:
| ● | 671,853 shares of common stock issuable upon the exercise of stock options outstanding as of June 30, 2016 at a weighted-average exercise price of $14.99 per share; | |
| ● | 970,000 shares of common stock issuable upon the conversion of Series A Preferred Stock; | |
| ● | 758,347 shares of common stock issuable upon the exercise of outstanding warrants at a weighted-average exercise price of $18.21 per share; and | |
| ● | 681,878 shares of common stock reserved for future issuance under our 2014 Plan. |
| 50 |
Selected consolidated Financial Data
You should read the following selected historical consolidated financial data below together with “Capitalization,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements, related notes and other financial information included elsewhere in this prospectus. The selected financial data in this section are not intended to replace the financial statements and are qualified in their entirety by the financial statements and related notes included elsewhere in this prospectus. The following selected statements of operations data for the six months ended June 30, 2016 and 2015, and the balance sheet data as of June 30, 2016 are derived from our unaudited condensed financial statement, and for the years ended December 31, 2015 and 2014 and the balance sheet data as of December 31, 2015 and 2014, are derived from our audited financial statements appearing elsewhere in this prospectus.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
| SIX
MONTHS ENDED JUNE 30, |
YEAR ENDED DECEMBER 31, |
|||||||||||||||
| 2016 | 2015 | 2015 | 2014 | |||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
| Operating costs and expenses: | ||||||||||||||||
| Research and development | (2,634,494 | ) | (1,590,823 | ) | $ | (3,434,016 | ) | $ | (6,323,896 | ) | ||||||
| IPR&D expense | (39,500,000 | ) | - | - | - | |||||||||||
| General and administrative | (2,980,043 | ) | (1,738,625 | ) | (6,388,000 | ) | (6,600,870 | ) | ||||||||
| Loss from operations | (45,114,537 | ) | (3,329,448 | ) | (9,822,016 | ) | (12,924,766 | ) | ||||||||
| Other income (expense): | ||||||||||||||||
| Change in fair value of derivative liability | 1,905,289 | – | (2,125,117 | ) | – | |||||||||||
| Loss on issuance of hybrid debt instrument | (1,584,218 | ) | - | - | - | |||||||||||
| Loss on conversion of debt | (6,187,337 | ) | - | - | - | |||||||||||
| Loss on disposal of subsidiaries | - | – | – | (1,069,675 | ) | |||||||||||
| Other expense | (13,551 | ) | (225,515 | ) | (295,033 | ) | (326,916 | ) | ||||||||
| Interest income | 27 | 1,088 | 1,694 | 18,959 | ||||||||||||
| Interest expense | (348,470 | ) | (2,512 | ) | (266,999 | ) | (4,706 | ) | ||||||||
| (6,228,260 | ) | (226,939 | ) | (2,685,455 | ) | (1,382,338 | ) | |||||||||
| Net Loss | (51,342,797 | ) | (3,556,387 | ) | (12,507,471 | ) | (14,307,104 | ) | ||||||||
| Other Comprehensive Loss from Foreign Currency Translation Adjustment | – | (327,054 | ) | (321,942 | ) | (324,578 | ) | |||||||||
| Total Comprehensive Loss | $ | (51,342,797 | ) | $ | (3,883,441 | ) | $ | (12,829,413 | ) | $ | (14,631,682 | ) | ||||
| Net loss per share of common stock, basic and diluted* | $ | (8.28 | ) | $ | (0.84 | ) | $ | (3.02 | ) | $ | (3.55 | ) | ||||
| Weighted-average shares of common stock outstanding, basic and diluted* | 6,197,776 | 4,221,328 | 4,254,470 | 4,118,062 | ||||||||||||
_______________________
*See Note 2 to our audited financial statements included elsewhere in this prospectus for an explanation of the calculations of our net loss per share—basic and diluted, and the weighted-average shares of common stock outstanding—basic and diluted.
| 51 |
CONSOLIDATED BALANCE SHEETS
| AS OF JUNE 30, 2016 | AS OF DECEMBER 31, | |||||||||||
| Unaudited | 2015 | 2014 | ||||||||||
| ASSETS | ||||||||||||
| Current Assets: | ||||||||||||
| Cash | 240,786 | $ | 132,229 | $ | 2,507,401 | |||||||
| Restricted Cash | 66,510 | 66,510 | 66,000 | |||||||||
| Prepayment on Acquisition | 3,744,517 | – | ||||||||||
| Prepaid Expenses and Other | 171,199 | 247,298 | 204,012 | |||||||||
| Total Current Assets | 478,495 | 4,190,554 | 2,777,413 | |||||||||
| Property and Equipment, Net | 58,471 | 62,021 | 119,449 | |||||||||
| Goodwill | 3,283,379 | 3,283,379 | 3,465,157 | |||||||||
| Indefinite-Lived Intangible Assets | 9,243,128 | 9,243,128 | 9,754,857 | |||||||||
| Other Assets | 97,238 | 129,306 | 199,270 | |||||||||
| Total Assets | 13,160,711 | $ | 16,908,388 | $ | 16,316,146 | |||||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||||||
| Current Liabilities: | ||||||||||||
| Accounts Payable | 2,021,205 | $ | 1,788,521 | $ | 852,760 | |||||||
| Accrued Expenses | 1,607,125 | 1,487,046 | 1,409,691 | |||||||||
| Hybrid Debt Instrument, Net | 3,652,749 | – | ||||||||||
| Other Current Liabilities | 19,647 | 19,098 | 41,472 | |||||||||
| Loans Due to Related Parties | 152,529 | 395,000 | 395,000 | |||||||||
| Total Current Liabilities | 3,800,506 | 7,342,414 | 2,698,923 | |||||||||
| Deferred Tax Liability | 2,918,518 | 2,918,518 | 3,080,097 | |||||||||
| Other Liabilities | 29,446 | 38,791 | 56,383 | |||||||||
| Total Liabilities | 6,748,470 | 10,299,723 | 5,835,403 | |||||||||
| Commitments and Contingent Liabilities(1) | – | – | ||||||||||
| Stockholders' Equity: | ||||||||||||
| Common Stock, $0.001 par value; 45,454,546 shares authorized as of June 30, 2016 and December 31, 2015 and 6,528,970 shares authorized as of December 31, 2014; 9,348,757 and 4,909,685 shares issued as of June 30, 2016 and December 31, 2015, respectively, and 4,545,213 shares issued and outstanding as of December 31, 2014; | 9,348 | 4,909 | 4,545 | |||||||||
| Additional Paid in Capital | 150,905,035 | 99,763,101 | 90,806,130 | |||||||||
| Accumulated Deficit | (139,474,696 | ) | (88,131,899 | ) | (75,624,428 | ) | ||||||
| Accumulated Other Comprehensive Income | 253,734 | 253,734 | 575,676 | |||||||||
| Treasury Stock | (5,281,180 | ) | (5,281,180 | ) | (5,281,180 | ) | ||||||
| Total Stockholders' Equity | 6,412,241 | 6,608,665 | 10,480,743 | |||||||||
| Total Liabilities and Stockholders' Equity | $ | 13,160,711 | $ | 16,908,388 | $ | 16,316,146 | ||||||
__________________
| (1) | In August 2013, we entered into an agreement to lease office and laboratory space in Lexington, Massachusetts under an operating lease with a commencement date of January 1, 2014 and a termination date of January 31, 2019. With the execution of this lease, we are required to maintain a $66,000 letter of credit as a security deposit, which is classified as a current asset within the consolidated balance sheet. In connection with the Lexington lease, we recorded $76,107 as prepaid rent as of June 30, 2016, with $54,012 recorded as a non-current asset and recorded $90,838 as prepaid rent as of December 31, 2015, with $61,377 recorded as a non-current asset. We also incurred a liability of $89,074 with respect to our contribution to the landlord’s leasehold improvements, of which $47,743 is outstanding as of June 30, 2016, with $29,446 recorded as a non-current liability, and $56,538 was outstanding as of December 31, 2015, with $38,791 recorded as a non-current liability. This liability is repayable as additional rent expense over the term of the lease and bears interest at 6%. In addition, we leased office space in London, U.K. during 2014 and 2015. The U.K. lease was terminated in March 2015 in accordance with the terms of the lease. |
| 52 |
Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read together with our “Selected Financial Data” and our financial statements, related notes, and other financial information included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those described in, or implied by, the forward-looking statements. Factors that could cause or contribute to those differences include, but are not limited to, those identified below and those discussed above in the section entitled “Risk Factors.”
BUSINESS OVERVIEW
We are a clinical-stage biopharmaceutical company focused on discovery, research and development of next-generation biologic drugs and novel orphan oncology therapeutics that may contribute to improvements in global human health. Our 200+ patent portfolio covers next generation biologic drugs and novel oncology therapeutics and provides protection for our current drug candidates and positions as well as strategic partnership and commercialization opportunities.
Our objective is to leverage our portfolio to maximize out-license opportunities that generate working capital to both build incremental shareholder value and provide funding necessary to clinically develop our orphan oncology drug candidate pipeline through to market launch.
Our lead product candidates include ErepoXen, a polysialylated form of erythropoietin (EPO) for the treatment of anemia in pre-dialysis patients with chronic kidney disease, and FDA orphan designated oncology therapeutics Virexxa and OncoHist for the treatment of progesterone receptor negative endometrial cancer and refractory acute myeloid leukemia, respectively.
We incorporate our patented and proprietary technologies into a number of drug candidates currently under development either in-house or with biotechnology and pharmaceutical collaborators in order to create what we believe will be the next-generation biologic drugs and therapeutics. While we primarily focus on researching and developing orphan oncology drugs, we also have significant interests in drugs being developed by our collaborators to treat, among others, hemophilia and anemia. Our four core proprietary technologies are:
| PolyXen | An enabling biological platform technology designed to extend the circulation in the human body for a variety of existing drug molecules and, thereby, to create potentially superior next generation drug candidates. PolyXen is based on the concept of polysialylation and utilizes polysialic acid, or PSA, which is a biopolymer, comprising a chain of sialic acid molecules. PSA is a natural constituent of the human body, though we obtain our PSA from a bacterial source. |
| Virexxa | A small molecule therapeutic with the potential to confer sensitivity to cancer cells to hormone therapeutics that are otherwise insensitive to such treatments. Virexxa, sodium cridanimod, belongs to a class of low-molecular weight synthetic interferon inducers. In addition to its immunomodulatory properties, Virexxa has been shown to increase levels of progesterone receptor expression in tumor tissue of patients who are progesterone receptor deficient, and thus may restore sensitivity of non-responsive endometrial cancers to hormonal (e.g., progestin) therapy. Based on preclinical observations, Virexxa may also be therapeutically relevant in other hormone-resistant cancers, such as triple-negative breast cancer. Virexxa has been granted an Orphan Drug Designation by the FDA, for treatment of progesterone receptor negative endometrial cancer in conjunction with progesterone therapy. |
| OncoHist | A novel therapeutic platform technology that utilizes the properties of modified human histone H1.3 for targeted cell necrosis or apoptosis programed cell death, which may enable OncoHist to treat a broad range of cancer indications. OncoHist, unlike many competing oncology therapies, is based on a molecule occurring naturally in the human body, in the cell nucleus, and is therefore expected to be less toxic and immunogenic than other oncology therapies. |
| ImuXen | A novel liposomal co-entrapment encapsulation technology designed to maximize both cell and immune system mediated responses. The technology is based on the co-entrapment of the nominated antigen(s) in a liposomal vesicle. The technology when applied may create new vaccines and improve the use and efficacy of certain existing human vaccines. |
| 53 |
These proprietary technologies may address unmet needs, improve the performance of existing drugs, and create new patentable drug candidates. All of our drug candidates are in the development stage and none has yet received regulatory approval for marketing in the U.S. by the FDA or by any applicable agencies in other countries.
As described in their audit report, our auditors have included an explanatory paragraph that states that we have incurred recurring losses and negative cash flows from operations since inception and have an accumulated deficit at December 31, 2015 of $88.1 million and $3.4 million of indebtedness. These matters raise substantial doubt about our ability to continue as a going concern. Our financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
Critical Accounting Estimates
The preparation of our financial statements in conformity with U.S. GAAP requires management to make estimates, judgments and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amount of expenses during the reporting period. On an ongoing basis, we evaluate our estimates that are based on historical experience and on various assumptions that we believe to be reasonable under the circumstances. The result of these evaluations forms the basis for making judgments about the carrying values of assets and liabilities and the reported amount of expenses that are not readily apparent from other sources. Because future events and their effects cannot be determined with certainty, actual results could differ from our assumptions and estimates, and such differences could be material.
Management believes that the following accounting estimates are the most critical to aid in fully understanding and evaluating our reported financial results, and they require management’s most difficult subjective or complex judgments, resulting from the need to make estimates about the effect of matters that are inherently uncertain. The following narrative describes these critical accounting estimates, the judgments and assumptions and the effect if actual results differ from these assumptions.
Revenue Recognition
We derive our revenue from our license and collaboration arrangements with pharmaceutical and biotechnology partners, some of which include royalty agreements based on potential net sales of approved commercial pharmaceutical products. Revenue from our collaborative partners are generally paid directly by the partners and are recognized on the accrual basis when all the following criteria are met: (i) persuasive evidence of an arrangement exists, (ii) delivery (or passage of title) has occurred or services have been rendered, (iii) the seller's price to the buyer is fixed or determinable, and (iv) collectability is reasonably assured.
The terms of our license agreements include delivery of an intellectual property license to a collaboration partner. We may be compensated under license arrangements through a combination of non-refundable upfront receipts, development and regulatory objective receipts and royalty receipts on future product sales by partners. We make our best estimate of the period over which we expect to fulfill our performance obligations, which may include technology transfer assistance, research activities, clinical development activities, and manufacturing activities from development through the commercialization of the product. Given the uncertainties of these collaboration arrangements, significant judgment is required to determine the duration of the performance period.
Non-refundable upfront license fees received, whereby our continued performance or future obligations are considered inconsequential or perfunctory to the relevant licensed technology, are recognized as revenue upon delivery of the technology in accordance with U.S. GAAP. This determination requires significant judgment to assess the nature of any continuing obligations. Reimbursements for research and development services completed by us related to the collaboration agreements are recognized in operations as revenue on a gross basis.
We expect to receive royalty receipts in the future as products are sold. We expect to recognize royalty revenue in the period of sale, based on the underlying contract terms, provided that the reported sales are reliably measurable and we have no remaining performance obligations, assuming all other revenue recognition criteria are met.
Our license and collaboration agreements with certain collaboration partners could also provide for future receipts to us based solely upon the performance of the respective collaboration partner in consideration of milestone extensions or upon the achievement of specified sales volumes of approved drugs. For such receipts, we expect to recognize the receipts as revenue when earned under the applicable contract terms on a performance basis or ratably over the term of the agreement. These receipts may also be recognized as revenue when our continued performance or future obligations are considered inconsequential or perfunctory.
| 54 |
Embedded Derivatives Related to Debt Instruments
In our financing arrangements, we issue debt instruments that may include features that meet the criteria of embedded derivatives requiring bifurcation. The fair value of each embedded derivative is valued independently using a “with-and-without” method. The “with-and-without” methodology involves valuing the whole instrument on an as-is basis and then valuing the instrument without the individual embedded derivative. The difference between the entire instrument with all of the embedded derivatives compared to the instrument without the individual embedded derivative is the fair value of that individual derivative. The embedded derivatives are settled when the underlying debt instrument is settled. Therefore, there are three possible settlement mechanisms: the debt instrument can be converted into equity, repaid early, or held to maturity.
In connection with our July 2015 financing, we developed a set of potential outcomes resulting in the settlement of the SPA Note consisting of a future qualifying capital raise with conversion, default of the SPA Note, the SPA Note being converted to equity and the SPA Note being held to maturity. These were included in a valuation model utilizing Monte Carlo Simulations to develop the fair value of the embedded derivatives, which included a simulation of the Company’s stock price with consideration provided for the expected volatility of the Company, the expected life of the host instrument, and risk free rate. The assumptions used in calculating the fair value represents our best estimates and involves inherent uncertainties and the application of our judgment. As a result, the use of alternate assumptions would result in outcomes that could be materially different. Additionally, the Company is required to update its assumptions and estimates at each valuation date. Based on updated circumstances, factors and knowledge of the Company at future valuation dates, then applicable assumptions and estimates could result in material changes in the estimated fair value of the embedded derivatives.
Share-Based Payments
Share-based payments includes grants of options to employees and nonemployees to purchase shares of common stock, grants of Joint Share Ownership Plan (JSOP) awards to employees, as well as agreements to issue common stock in exchange for services provided by nonemployees. Total share-based compensation related to stock options, common stock awards, and warrants was $2,018,263 and $237,198 during the six months ended June 30, 2016 and 2015, respectively.
Currently, we utilize one option plan, the Xenetic Biosciences, Inc. Equity Incentive Plan pursuant to which we may grant options to purchase shares of common stock to employees and nonemployees. Prior to the acquisition of Xenetic U.K. in January 2014, the Company had two option plans, the Lipoxen plc Unapproved Share Option Plan and the Xenetic Biosciences plc 2007 Share Option Scheme. Both of these plans were converted subsequent to year end to reflect the new shares of common stock issued related to the Acquisition. As part of the conversion, option holders under both plans have the right to subscribe for a number of shares of common stock in exchange for the cancellation and surrender by the option holder in a manner similar to which the shareholders prior to the Acquisition were given the right to acquire shares of common stock in the new company according to the terms of the Acquisition.
We measure share-based payments to employees in accordance with Financial Accounting Standards Board Accounting Standards Codification (ASC) Topic 718, Compensation – Stock Compensation and to nonemployees in accordance with ASC Topic 505, Equity. Stock option compensation expenses are based on the estimated fair value of the underlying option calculated using the Black-Scholes option pricing model, which requires the input of subjective assumptions and judgments, including estimating share price volatility and expected term of the awards. Our shares do not have a sufficient trading history for us to adequately assess the fair value of the stock option grants. Therefore, for all share-based payments, we determine the expected volatility based on a weighted-average of the historical volatility of a peer group of comparable publicly traded companies with product candidates in similar stages of development to our product candidates in conjunction with our historical volatility. We intend to consistently apply this methodology of using a peer group of comparable companies until the historical volatility of our own share price is relevant to measure expected volatility for future equity based awards. For employee stock options issued in 2014 that qualify as “plain vanilla” stock options in accordance with Staff Accounting Bulletin No. 110 (SAB 110) issued by the SEC, the expected term is estimated using the simplified method, as defined in SAB 110. The Company has a limited history of stock option exercises, which does not provide a reasonable basis for the Company to estimate the expected term of employee stock options. For all other employee stock options, we estimate the expected life using judgment based on the anticipated research and development milestones of the Company’s clinical projects and behavior of the Company’s employees. The expected life of nonemployee options is the contractual life of the option. The assumptions used in calculating the fair value of the stock option grants represent our best estimates and involve inherent uncertainties and the application of our judgment. As a result, if factors change and we use different assumptions, share-based payments expense could be materially different in the future.
| 55 |
For employee options that vest based solely on service conditions, the fair value measurement date is generally on the date of grant and the related compensation expense, less expense for expected forfeitures, is recognized on a straight-line basis over the requisite vesting period of the awards. For nonemployee options, the fair value measurement date is the earlier of the date the performance of services is complete or the date the performance commitment has been reached. We generally determine that the fair value of the stock options is more reliably measurable than the fair value of the services received. Compensation expense related to stock options granted to nonemployees that vest based solely on service conditions is subject to re-measurement at each reporting period until the options vest and is recognized on a straight-line basis over the estimated vesting period of the awards.
We estimate forfeitures at the time of grant and revise those estimates in subsequent periods if actual forfeitures differ from those estimates. During 2015 and 2014, we applied a forfeiture rate of 0% as we have not historically experienced forfeitures. Upon exercise, stock options are redeemed for newly issued shares of common stock.
The fair value of common stock awards issued in exchange for services provided by nonemployees is generally determined by using the fair value of the services provided, as this provides the most reliable measure of the fair value of the awards. Share-based payments expense is recognized as services are rendered on a straight-line basis. The assumptions used in calculating the fair value of the common stock awards represent our best estimates and involve inherent uncertainties and the application of our judgment. As a result, if factors change and we use different assumptions, share-based payments expense related to the common stock awards could be materially different in the future.
Under the JSOP, our shares are jointly purchased at fair market value by the participating executives and the trustees of the JSOP trust, with shares held in the JSOP trust. For U.S. GAAP purposes the awards are valued as employee options. The JSOP trust holds the shares of the JSOP until such time as the JSOP shares are vested and the participating executives exercise their rights under the JSOP. The JSOP trust is granted an interest bearing loan by us in order to fund the purchase of its interest in the JSOP shares. The loan held by the trust is eliminated on consolidation in the financial statements of the Company. We funded portion of the share purchase price is deemed to be held in treasury until such time as they are transferred to the employee and is recorded as a reduction in equity.
The exercise price of the JSOP “option” is deemed to be the market value of the shares at the date of issue. The awards vest based on certain market conditions, which require each tranche of shares to meet specific market share price hurdles, or change in control conditions, as defined by the plan. Under the JSOP and subject to the vesting of the participants’ interest, participating executives will, when the JSOP shares are sold, be entitled to a share of the proceeds of sale equal to the growth in market value of the JSOP shares versus the exercise price, less simple interest on the original share purchase price, net of executives’ cash contribution at inception, as agreed for each grant (Carry Charge). The balance of the proceeds will remain to the benefit of the JSOP trust and be applied to the repayment of the loan originally made by us to the JSOP trust. Any funds remaining in the JSOP trust after settlement of the loan and any expenses of the JSOP trust are for our benefit.
We measure the fair value of JSOP awards using Monte Carlo simulations, which requires estimates based on our judgment, as well as other assumptions. These estimates include the expected term of each tranche of the JSOP awards, which we determine to be the initial life of the awards, and expected volatility, which is based on a weighted average of the historical volatility of a peer group of comparable publicly traded companies with product candidates in similar stages of development to our product candidates in conjunction with the historical volatility of Xenetic Biosciences plc’s shares when traded on the U.K. AIM market. We have applied an expected dividend yield of 0% as we have not historically declared a dividend and do not anticipate declaring a dividend during the expected life of the awards. The risk-free rate is based upon the U.S. Treasury yield curve in effect at the time of grant, with a term that approximates the expected life of the awards. The compensation expense is recorded over the expected life of the option, regardless of whether the awards vest. Having established the full value of the JSOP awards using the Monte Carlo simulation outlined above, a deduction is made in respect of the anticipated Carry Charge in order that the expense recorded in the financial statements only represents the participating executives’ net interest in the awards. The assumptions used in calculating the fair value of the JSOP awards represent our best estimates and involve inherent uncertainties and the application of our judgment. As a result, if factors change and we use different assumptions, share-based payments expense related to the JSOP awards could be materially different in the future.
On exercise of the JSOP awards by the executives the Carry Charge due to the Company will be recognized as additional paid-in capital, arising from the sale of treasury stock.
| 56 |
Warrants
In connection with certain financing, consulting and collaboration arrangements, we issue warrants to purchase shares of the Company’s common stock. Outstanding warrants are standalone instruments that are not puttable or mandatorily redeemable by the holder and are classified as equity awards. We measure the fair value of the awards using the Black-Scholes option pricing model, which requires the input of subjective assumptions and judgments, including estimating the expected term of the awards and the share price volatility, at each reporting period until the measurement date is reached. The expected term is deemed to be the contractual life of the warrant and we determine the expected volatility based on a weighted-average of the historical volatility of a peer group of comparable publicly traded companies with product candidates in similar stages of development to our product candidates in conjunction with our historical volatility. As of June 30, 2016, and December 31, 2015, warrants to purchase 758,347 shares of common stock were outstanding.
All other warrants are recorded at fair value as compensation expense on a straight-line basis over the requisite service period or at the date of issuance, if there is not a service period or if service has already been rendered. For warrants that contain vesting triggers based on the achievement of certain objectives, we apply judgment to estimate the probability and timing of the achievement of those objectives. These estimates involve inherent uncertainties, and as a result, if the probability or timing of the achievement of those objectives change, expense related warrants could be materially different in the future.
Warrants issued to collaboration partners in conjunction with the issuance of common stock are recorded at fair value as a reduction in additional paid-in capital of the common stock issued.
Goodwill and Indefinite-lived Intangible Assets
Goodwill
Goodwill is not amortized but is reviewed for impairment annually as of October 1, or when events or changes in the business environment indicate that all, or a portion, of the carrying value of the reporting unit may no longer be recoverable. Under this method, we compare the fair value of our reporting unit to its carrying value. If the fair value is less than the carrying amount, a more detailed analysis is performed to determine if goodwill is impaired. An impairment loss, if any, is measured as the excess of the carrying value of goodwill over the fair value of goodwill. We also have the option to first assess qualitative factors to determine whether the existence of events or circumstances leads us to determine that it is more likely than not (that is, a likelihood of more than 50%) that goodwill is impaired. If we choose to first assess qualitative factors and it is determined that it is not more likely than not goodwill is impaired, we are not required to take further action to test for impairment. We also have the option to bypass the qualitative assessment and perform only the quantitative impairment test, which we may choose to do in some periods but not in others. As the option to perform the qualitative assessment is not a permanent election, we reassess this option during each annual impairment review.
We determine our reporting unit by identifying the components of our operating segment with similar economic characteristics based on quantitative and qualitative factors that have discrete financial information available. We determined that we have one reporting unit as of October 1, 2015 and 2014, the dates of our annual impairment reviews. Based on our annual impairment reviews, we used the quantitative method and determined no adjustment to the carrying value of goodwill would be necessary as the fair value of our reporting unit significantly exceeded its respective carrying value as of October 1, 2015 and 2014, respectively. If the fair value of our reporting unit were to be reduced by one-half, the fair value would still significantly exceed the carrying value of the reporting unit at October 1, 2015. There can be no assurance that future events will not result in an impairment of goodwill.
Indefinite-lived Intangible Assets
Our indefinite-lived intangible assets consist of acquired IPR&D. IPR&D intangible assets are considered indefinite-lived intangible assets until completion or abandonment of the associated research and development efforts. IPR&D is not amortized but is reviewed for impairment annually as of October 1, or when events or changes in the business environment indicate the carrying value may be impaired. If the fair value of the intangible asset is less than the carrying amount, we perform a quantitative test to determine the fair value. The impairment loss, if any, is measured as the excess of the carrying value of the intangible asset over its fair value. We also have the option to first assess qualitative factors to determine whether the existence of events or circumstances leads us to determine that it is more likely than not (that is, a likelihood of more than 50%) that our indefinite-lived intangible asset is impaired. If we choose to first assess qualitative factors and it is determined that it is not more likely than not our indefinite-lived intangible asset is impaired, we are not required to take further action to test for impairment. We also have the option to bypass the qualitative assessment and perform only the quantitative impairment test, which we may choose to do in some periods but not in others. As the option to perform the qualitative assessment is not a permanent election, we reassess this option during each annual impairment review. During 2015 and 2014, we used the quantitative method and determined the fair value of the indefinite-lived intangible asset exceeded its carrying value as of October 1, 2015 and 2014.
| 57 |
Significant judgments are inherent in the calculation of fair value. With the assistance of an independent third-party, we calculated the fair value of our IPR&D by using the Multi-Period Excess Earnings Method (MPEEM) which is a form of the income approach. Under the MPEEM, the fair value of an intangible asset is equal to the present value of the asset’s incremental after-tax cash flows (excess earnings) remaining after deducting the market rates of return on the estimated value of contributory assets (contributory charge) over its remaining useful life. This method requires us to make long-term projections of the amount and timing of income and expenses related to development and commercialization of the acquired intangible asset and assumptions regarding the rate of return on contributory assets, the weighted average cost of capital and the discount rate for estimated future after-tax cash flows. Specifically, this method took into account our estimates of future incremental milestone payments that may be achieved upon completion of clinical trial stages, regulatory approval and sales goals upon commercialization, as well as our expected royalty income based on sales upon commercialization. Projected expenses are based on our forecasted spend required to complete the development of our IPR&D, which will require the Company to raise further capital to fund the development. Our projections are estimates subject to change based on several factors including the results of clinical trials and delays in regulatory approval. The discount rate used is commensurate with the uncertainties associated with the economic estimates described above and reflects the stage of development, the time and resources needed to complete the development of the product and the risks of advancement through regulatory approval processes.
Key assumptions utilized in the fair valuation of our indefinite-lived intangible asset OncoHist are as follows:
| · | Discount rate – 47.5% | |
| · | Weighted average cost of capital – 16.0% | |
| · | Estimated aggregate milestone receipts – approximately $300 million | |
| · | Royalty rates – 10% of net sales |
While we believe reasonable estimates and appropriate assumptions were utilized to calculate the fair value of OncoHist, it is possible a material change could occur. Use of different estimates and judgments could yield materially different results in our analysis and could result in materially different asset values or expense.
There can be no assurance that we will be able to successfully develop and complete the acquired IPR&D program and profitably commercialize the underlying product candidates before our competitors develop and commercialize similar products, or at all. Moreover, if the acquired IPR&D program fails or is abandoned during development, then we may not realize the value we have estimated and recorded in our financial statements on the acquisition date, and we may also not recover the research and development investment made since the acquisition date to further develop that program. If such circumstances were to occur, our future operating results could be materially adversely impacted.
We did not record an impairment charge as a result of our goodwill or indefinite-lived intangible asset impairment tests in 2015 or 2014. We will continue to closely monitor the performance of our indefinite-lived intangible asset and reporting unit. If the business experiences adverse changes in our key assumptions and judgments, we will perform an interim goodwill and/or indefinite-lived intangible asset impairment analysis. There can be no assurance that future events will not result in an impairment of our goodwill or indefinite-lived intangible asset. As a result of the going concern uncertainty discussed under Liquidity and Capital Resources below, the recoverability and classification of the Company’s intangible assets and goodwill could be adversely affected.
| 58 |
RESULTS OF OPERATIONS
Comparison of Six Months Ended June 30, 2016 and 2015
The comparison of our historical results of operations for the six months ended June 30, 2016 to the six months ended June 30, 2015 is set forth below:
| Description | Six Months Ended June 30, 2016 | Six Months Ended June 30, 2015 | Increase (Decrease) | Percentage Change | ||||||||||||
| Research and development | $ | (2,634,494 | ) | $ | (1,590,823 | ) | $ | (1,043,671 | ) | (66 | ) | |||||
| IPR&D expense | (39,500,000 | ) | – | (39,500,000 | ) | – | ||||||||||
| General and administrative | (2,980,043 | ) | (1,738,625 | ) | (1,241,418 | ) | (71 | ) | ||||||||
| Loss from operations | (45,114,537 | ) | (3,329,448 | ) | (41,785,089 | ) | (1,255 | ) | ||||||||
| Other income (expense) | (13,551 | ) | (225,515 | ) | 211,964 | 94 | ||||||||||
| Change in fair value of derivative liability | 1,905,289 | – | 1,905,289 | – | ||||||||||||
| Loss on issuance of hybrid debt instrument | (1,584,218 | ) | – | (1,584,218 | ) | – | ||||||||||
| Loss on conversion of debt | (6,187,337 | ) | – | (6,187,337 | ) | – | ||||||||||
| Interest income | 27 | 1,088 | (1,061 | ) | (98 | ) | ||||||||||
| Interest expense | (348,470 | ) | (2,512 | ) | (345,958 | ) | (13,772 | ) | ||||||||
| Net loss | $ | (51,342,797 | ) | $ | (3,556,387 | ) | $ | (47,786,410 | ) | (1,344 | ) | |||||
Research and Development
Overall, corporate R&D expenses for the six months ended June 30, 2016 increased by $40.5 million, or 2,549% to $42,134,494 from $1,590,823 in the comparable period in 2015. The table below sets forth the R&D costs incurred by the Company, by category of expense, for the six months ended June 30, 2016 and 2015:
| Six Months Ended, | ||||||||
| Category of Expense | June 30, 2016 | June 30, 2015 | ||||||
| IPR&D expense | $ | 39,500,000 | $ | – | ||||
| Outside services and Contract Research Organizations | 986,750 | 1,029,537 | ||||||
| Salaries and wages | 248,792 | 267,481 | ||||||
| Share-based compensation expense | 1,234,240 | 150,881 | ||||||
| Rent | 44,441 | 44,913 | ||||||
| Other | 120,271 | 98,011 | ||||||
| Total research and development expense | $ | 42,134,494 | $ | 1,590,823 | ||||
The increase in R&D expenses during the six months ended June 30, 2016, compared to the same period in 2015 was primarily due to the Company’s acquisition and immediate expensing of the Virexxa asset ($39.5 million) coupled with $1.36 million recognized in connection with warrants issued to Serum. Separate from these, R&D costs were relatively flat period over period.
General and Administrative
General and administrative expenses increased by $1,241,418 or 71% for the six months ended June 30, 2016 to $2,980,043 from $1,738,625 in the comparable period of 2015. The most significant drivers of the change were related to an increase of approximately $909,000 in legal and consulting services in connection with our efforts to effect our planned capital stock offering and uplist to a national exchange as well as related accounting and regulatory costs. The increase is also driven by approximately $436,000 increase in share-based compensation. These increases were partially offset by decreases in personnel and other administrative costs.
| 59 |
Hybrid Debt Instrument
On July 1, 2015, the Company entered into a Securities Purchase Agreement (the “SPA”) with Pharmsynthez providing for the issuance of a minimum of a $3 million 10% Senior Secured Collateralized Convertible Promissory Note (the “SPA Note”). The SPA also provides for the issuance of certain warrants up to the amount of the SPA Note. The convertible debt and its embedded debt-like features were recorded on the face of the condensed consolidated balance sheet within current liabilities as an aggregate hybrid debt instrument.
On April 22, 2016, Pharmsynthez converted all convertible notes (in the principal amount of $6.5 million plus accrued interest of approximately $228,000), issued by the Company to Pharmsynthez in 2015 and 2016. The conversion rate was $4.95 per share. As such, the Company issued to Pharmsynthez 1,373,036 shares of common stock in connection with conversion of the convertible notes. The related embedded derivatives, which had been bifurcated from the host debt and accounted for separately, were settled by action of the conversion. The Company recognized a net loss on conversion, including a final mark-to-market of the compound derivative, of $4.4 million.
Other Expense
Other expense decreased approximately $211,964, or 94% to $13,551 for the six months ended June 30, 2016 from $225,515 in the comparable period in 2015. This decrease is primarily related to effects on payables held in foreign currency compared to the same period in 2015 which also included adjustments to foreign currency translation for prior period corrections.
Interest Expense
Interest expense increased from $2,512 to approximately $348,000 for the six months ended June 30, 2016 compared to the same period in 2015. The increase in interest expense is primarily due to interest charges associated with the SPA Note and APA Note. There was not a similar promissory note in the comparable period in 2015. The interest expense for the six months ended June 30, 2015, is primarily related to a financing arrangement with the landlord of the Company’s office and lab lease in the US, which commenced in January 2014.
Liquidity and Capital Resources
At June 30, 2016 and December 31, 2015, we had working capital deficits of approximately $3.3 million and $3.2 million, respectively. At June 30, 2016 we had approximately $0.2 million in cash and $3.6 million in accounts payable and accrued expenses. At December 31, 2015, we had approximately $0.13 million in cash and $3.3 million in accounts payable and accrued expenses. Our working capital has increased in 2016 due primarily to $3.5 million of debt proceeds offset by $3.4 million net cash used during the six months end June 30, 2016, which consisted of meeting creditor obligations, furthering our clinical development, and other general operating needs.
As of August 15, 2016, the Company will be required to raise additional working capital in order to meet its financial obligations for the next 12 months.
We have historically relied upon equity financing to fund our operations. Since 2005, we have raised approximately $53.5 million in equity financing, including $6.5 million from the April 2016 conversion of the SPA Note and APA Note to equity, $10 million from the sale of shares to Baxter in January 2014, as well as received $10 million from revenue producing activities in the years prior to 2014. Approximately 90% of that revenue was from a single customer, Baxter, in connection with milestone receipts and fees for services. We expect the majority of our funding through equity or equity linked instruments to continue as a trend for the foreseeable future.
On July 1, 2015, the Company entered into the SPA with Pharmsynthez for the issuance of the SPA Note, which provided net proceeds of approximately $3 million in July 2015 for the general working capital needs of the Company.
| 60 |
In November 2015 we entered into the APA which included the 1st amendment to the SPA (the “Amended SPA”) wherein Pharmsynthez agreed to purchase from the Company up to $3.5 million of additional 10% Convertible Promissory Notes (the “APA Notes”). The APA contains a total financing commitment from Pharmsynthez in the amount of $10 million. The APA Notes represent bridge financing to be drawn down from this $10 million. As of August 15, 2016, the Company had received net proceeds of $4.0 million from the APA Notes, leaving a balance of $6.0 million in funding commitment from Pharmsynthez.
In connection with the closing of the APA in April 2016, the Company issued 3,045,455 shares of its common stock to Pharmsynthez. In addition, Pharmsynthez converted all convertible notes (in the principal amount of $6.5 million plus accrued interest of approximately $300,000), issued by the Company to Pharmsynthez in 2015 and 2016. The conversion rate as set forth in the notes was $4.95 per share. As such, the Company issued to Pharmsynthez 1,373,036 shares of its common stock in connection with conversion of the convertible notes, which amount, together with the 3,045,455 shares of common stock in connection with the closing of the Asset Purchase Agreement, resulted in an aggregate of 4,418,491 new shares of common stock being issued to Pharmsynthez.
Pharmsynthez, as part of the APA, has agreed to invest $6.0 million (the “Additional Investment”) as part of our planned total capital raise and planned up-list to a national securities exchange (the “Capital Raise”). The total Capital Raise of $10 million will consist of $6.0 million in the Additional Investment (of which $0.5 million has been received) and $4.0 million in proceeds resulting from the general public offering. The Company believes this total financing plus the anticipated milestone payment in 2017 will be sufficient for the Company to meet its financial obligations and continue operations for the next 12 months. If the Company fails to achieve the milestone, the Company will need to raise an additional $3 million to fund our basic operations for the next 12 months. Although the Company is optimistic, the achievement of this milestone payment is not guaranteed.
In the event that the Company is unable to cause a listing of its securities on the NASDAQ Capital Markets, then, pursuant to the APA, Pharmsynthez shall loan to the Company up to the Additional Investment of $6.0 million on essentially similar terms as the APA Notes. This outcome would require the Company to seek additional financing and/or defer certain research and development activities in order to meet its financial obligations over the next 12 months.
Until we reach commercialization of our technology or receive significant and regular cash flows from our current collaborations or from planned out-licensing of our technology, we expect the trend of accessing capital markets to finance our working capital needs to continue.
The only significant cash receipts that we could expect from our current collaborations would be from Shire. Due to the uncertainties and risks inherent in the clinical development process, we are unable to predict precisely when those receipts may occur, if ever. We do not expect any significant receipts to become due within the next three months. However, there can be no assurance that future receipts will ever become due because they are contingent on positive outcomes from Shire’s clinical development efforts in connection with the Factor VIII drug candidate.
We have commenced the process of seeking out-license arrangements for our ErepoXen™ technology but are currently unable to reliably predict when that process may result in an agreement. Due to the uncertainties inherent in the clinical research process and unknown future market conditions, there can be no assurance our ErepoXen™ technology will lead to any future income.
| 61 |
Cash Flows Used in Operating Activities
Cash flows used in operating activities for the six months ended June 30, 2016 totaled approximately $3.1 million, which includes a net loss of approximately $51.3 million offset by approximately $48.2 million in non-cash charges related to the Virexxa asset acquisition, which was immediately expensed ($39.5 million), as well as the hybrid debt instrument ($6.2 million including issuance loss, interest, amortization, change in fair value, and loss on extinguishment upon conversion of the debt host). In addition, as the Company recognized a net non-cash charge of approximately $2.0 million for share-based compensation and warrants.
Cash flows used in operating activities for the six months ended June 30, 2015 totaled approximately $2.2 million, which includes a net loss of approximately $3.6 million, partially offset by approximately $0.7 million in net decreases in account receivable and increase in accounts payable and accrued expenses, approximately $0.3 million in foreign exchange translation charges and approximately $0.2 million in non-cash charges for share-based compensation. The $2.2 million includes cash expenses of approximately $0.6 million in salaries, wages, employee fringe benefits and related taxes, including scientific staff, approximately $0.8 million in program-specific clinical development costs, $0.4 million in legal fees and $0.1 million in accounting and tax consultants.
Cash Flows from Investing Activities
For the six months ended June 30, 2016 and 2015, respectively, there were no significant cash sources or uses from investing activities.
Cash Flow from Financing Activities
The Company received $3.5 million in proceeds from issuance of $3.5 million 10% convertible secured promissory notes in connection with the APA. For the quarter ended June 30, 2015, there were no significant cash sources or uses from financing activities.
Off Balance Sheet Arrangements
The Company has no off balance sheet financing arrangements. The Company has one facility lease obligation and written employment agreements with three key employees as of June 30, 2016.
Recent Accounting Pronouncements
There has been no material change to the recent accounting pronouncements under consideration since those described in our Annual Report on Form 10-K filed on March 30, 2016.
Comparison of Year Ended December 31, 2015 and 2014
The comparison of our historical results of operations for the year ended December 31, 2015 to the year ended December 31, 2014 is as follows:
| Description | 2015 | 2014 | Increase (Decrease) |
Percentage Change |
||||||||||||
| Operating Costs and Expenses: | ||||||||||||||||
| Research and Development | $ | 3,434,016 | $ | 6,323,896 | $ | (2,889,880 | ) | 45.7 | ||||||||
| General and Administrative | 6,388,000 | 6,600,870 | (212,870 | ) | 3.2 | |||||||||||
| Loss from Operations | (9,822,016 | ) | (12,924,766 | ) | (3,102,750 | ) | 24.0 | |||||||||
| Other Income (Expense): | ||||||||||||||||
| Change in Fair Value of Derivative Liability | (2,125,117 | ) | – | 2,125,117 | 100.0 | |||||||||||
| Loss on Disposal of Subsidiaries | – | (1,069,675 | ) | (1,069,675 | ) | 100.0 | ||||||||||
| Other Expense | (295,033 | ) | (326,916 | ) | (31,833 | ) | 9.8 | |||||||||
| Interest Income | 1,694 | 18,959 | (17,265 | ) | 91.1 | |||||||||||
| Interest Expense | (266,999 | ) | (4,706 | ) | 262,293 | 5,573.6 | ||||||||||
| (2,685,455 | ) | (1,382,338 | ) | 1,303,117 | 94.3 | |||||||||||
| Net Loss | $ | (12,507,471 | ) | $ | (14,307,104 | ) | $ | (1,799,633 | ) | 12.6 | ||||||
| 62 |
Revenue
We did not record any revenues for the years ended December 31, 2015 and 2014.
Cost of Revenue
We did not incur any cost of revenue for the years ended December 31, 2015 and 2014.
Research and Development
We engage in independent research and development (R&D) in connection with its various technologies. Overall, corporate R&D expenses for the year ended December 31, 2015 decreased by approximately $2.89 million, or 45.7% to $3.43 million from $6.32 million in 2014. The table below sets forth the R&D costs incurred by the Company, by category of expense, for the years ended December 31, 2015 and 2014:
| Year ended December 31, | ||||||||
| Category of Expense | 2015 | 2014 | ||||||
| Outside Services and Contract Research Organizations | $ | 1,794,523 | $ | 4,296,795 | ||||
| Share-Based Expense | 886,805 | 952,829 | ||||||
| Salaries and Wages | 491,623 | 729,082 | ||||||
| Rents | 89,354 | 78,076 | ||||||
| Lab Consumables | 23,711 | 26,280 | ||||||
| Other | 148,000 | 240,834 | ||||||
| Total research and development expense | $ | 3,434,016 | $ | 6,323,896 | ||||
The decrease in R&D expenses during 2015 was primarily due to the planned deferral of IND-enabling preclinical work conducted in connection with the OncoHist program due to working capital constraints. The costs of conducting the ongoing ErepoXen human clinical trials in Australia, which are borne by the U.K. subsidiary Lipoxen, were relatively unchanged during 2015 as compared to 2014.
Research and Development by Category of Expense
Outside Services and Contract Research Organization Costs
The significant decrease in outside services and contract research organization costs of approximately $2.5 million, or 58.2% for the year ended December 31, 2015 is primarily due to the planned deferral of IND-enabling preclinical work conducted in connection with the OncoHist program due to working capital constraints. The costs of conducting the ongoing ErepoXen human clinical trials were relatively unchanged as the trials proceeded as planned, with costs of approximately $1.07 million and $1.12 million in 2015 and 2014, respectively.
Share-based Expense
Share-based expense decreased approximately $66,024 or 6.9% to $886,805 for the year ended December 31, 2015 from $952,829 for the prior year. The fluctuation is primarily due to the normal expensing of the fair value of stock option awards and warrants granted to R&D personnel in September 2015 and December 2014. The December 2014 warrant grants did not have a significant impact on the 2014 shared-based payments expense but resulted in a $706,500 expense for the year ended December 31, 2015. The 2014 share-based expense includes $811,196 for the fair value of common stock issued in exchange for an intellectual property assignment to the company made during 2014 compared to $0 for the comparable period in 2015.
| 63 |
Salaries and Wages
Salaries and wages decreased by approximately $237,000 or 32.6% to $491,623 for the year ended December 31, 2015 from $729,082 for the prior year. The decrease is due to the planned overall reduction in the number of scientific personnel following the closing of the U.K. lab facility. The layoffs of three U.K.-based scientific personnel at various points during 2014 were only partially offset by the hiring of two new scientists in the U.S. during the same year. The decrease is also partially related to certain non-recurring layoff costs incurred in 2014. There were no new R&D personnel hired in 2015.
Rents
Rent expense allocated to research and development increased approximately $11,000, or 14.1% to $89,354 from $78,076 for the year ended December 31, 2015 over the comparable period in 2014. During each period, we operated the same research and development facility, which shares its space with general and administrative employees. While the overall rent expense for this facility did not change during these periods, the expense allocated to research and development increased during the year ended December 31, 2015 due to a change in the Company’s method of allocation.
Lab Consumables
The slight decrease of approximately $2,000 in lab consumables expense is due to normal fluctuations in the amount of those supplies required for in-house research activities.
Other
Other expenses decreased approximately $93,000, or 38.5%, to $148,000 for the year ended December 31, 2015 from $240,834 for the prior year. The decrease in other expense results from the net aggregate change of all miscellaneous costs, including an approximately $36,000 decrease in computer and equipment costs, approximately $30,000 decrease in recruiting costs and approximately $30,000 decrease in general travel costs.
General and Administrative
General and administrative, or G&A, expenses decreased by approximately $213,000, or 3.2%, to $6,388,000 for the year ended December 31, 2015 from $6,600,870 for the prior year. Although the total level of general and administrative costs did not change significantly, there were significant changes of certain expenses as follows. Stock compensation expense increased approximately $1.04 million due to the normal expensing of the fair value of stock option awards granted to G&A personnel in September 2015 and December 2014. This increase was offset by a decrease in consulting, accounting and legal professional service costs of approximately $810,000 due to certain non-recurring costs during 2014 related to the Company’s transition to the U.S. as a U.S. public company and short term cost reduction initiatives. In addition, travel expenses and rent and utilities costs in 2015 decreased approximately $202,000 and $115,000, respectively, due to the closure of the U.K. office in March 2015.
All other general and administrative expenses resulted in a net decrease of approximately $121,000 for the year ended December 31, 2015 over the comparable period in 2014.
Change in Fair Value of Derivative Liability
The loss of approximately $2.1 million is recognized on the change in fair value of the Company’s compound derivative instrument during the year ended December 31, 2015. This change is primarily driven by the change in our stock price from period to period. The Company did not have debt instruments with embedded derivatives outstanding during the comparable period in 2014.
| 64 |
Loss on Disposal of Subsidiaries
The loss on disposal of subsidiaries is related to one transaction, the Hive Out Agreement, during the year ended December 31, 2014. There were no disposals of subsidiaries during the year ended December 31, 2015.
Other Income (Expense)
Other expense decreased approximately $32,000, or 9.8% to $295,033 for the year ended December 31, 2015 from $326,916 in 2014. This decrease is primarily related to decreased foreign currency transaction expenses following the change in functional currency of our foreign subsidiaries to the U.S. dollar in April 2015. This was offset by an approximately $60,000 loss recorded on the issuance of debt in July 2015.
Interest Income
Interest income decreased by approximately $17,000, or 91% to approximately $2,000 for the year ended December 31, 2015 from approximately $19,000 in 2014. The decrease is proportional to the decrease in average cash balances held by us during the period from January 1, 2014 to December 31, 2015 and is not due to any change in investment strategies.
Interest Expense
Interest expense increased by approximately $262,000, or 5,574%, to approximately $267,000 for the year ended December 31, 2015 from approximately $5,000 in 2014. The increase in interest expense is primarily due to interest charges associated with the SPA Note. There was not a similar promissory note in the comparable period in 2014. The Company also recognized interest expense related to a financing arrangement with the landlord of the Company’s office and laboratory lease in the U.S., which commenced in January 2014.
Liquidity and Capital Resources
At December 31, 2015 and 2014 we had working capital deficits of approximately $3.2 million and $78,000, respectively. At December 31, 2015, we had approximately $0.13 million in cash and $3.3 million in accounts payable and accrued expenses. At December 31, 2014 we had cash and accounts payable and accrued expenses of $2.5 million and $2.3 million, respectively. Our working capital has been reduced in 2015 due to our net loss of $12.5 million that includes $5.3 million net cash used in operating activities comprised of approximately $1.8 million in consulting, legal and other professional service fees, approximately $1.5 million in salaries and wages, including scientific staff, approximately $1.3 million in program-specific clinical development costs and approximately $232,000 in rent and utilities expenses. The $1.8 million in consulting, legal and other professional service fees cash outflows in 2015 includes $0.9 million of costs that were incurred during 2014 but paid in 2015. The $1.3 million applied to external research and development and clinical program costs primarily related to our ErepoXen drug candidate.
Cash Flows Used in Operating Activities
Cash flows used in operating activities for the year ended December 31, 2015 totaled approximately $5.3 million. The $5.3 million includes net operating cash uses of approximately $1.78 million in consulting, legal and other professional service fees, approximately $1.54 million in salaries and wages, including scientific staff, approximately $1.25 million in program-specific clinical development costs and approximately $232,000 in rent and utilities expenses.
Cash flows used in operating activities for the year ended December 31, 2014 totaled approximately $12.3 million. The $12.3 million includes net operating cash uses of approximately $7.00 million in consulting, legal and other professional service fees, approximately $3.01 million in salaries and wages, including scientific staff, and approximately $1.80 million in program-specific clinical development costs.
| 65 |
Cash Flows from Investing Activities
Cash flows used in investing activities for the year ended December 31, 2015 included approximately $2,000 from the purchase of assets consisting of laboratory equipment, offset by approximately $8,000 derived from the disposition of certain property and equipment during the year.
Cash flows used in investing activities for the year ended December 31, 2014 included approximately $58,000 from the purchase of assets consisting of office furniture and fixtures and laboratory equipment, partially offset by approximately $5,500 derived from the disposition of certain property and equipment during the year.
Cash Flow from Financing Activities
For the year ended December 31, 2015, we raised $3.0 million and $0.1 million with the issuances of the SPA Note and a short-term promissory note, respectively. From the proceeds of the SPA Note, we repaid our $0.1 million short-term promissory note.
For the year ended December 31, 2014 we received $10 million in proceeds in exchange for the issuance of approximately 10.7 million shares of common stock to Baxalta and we received approximately $102,000 in proceeds in connection with the exercise of stock options by the CEO of the company. The proceeds were applied toward our working capital needs during the year. During the year, we repaid approximately $286,000 on our loan to an affiliate of the Company.
Off Balance Sheet Arrangements
We have no off balance sheet financing arrangements. We have two facility lease obligations and written employment agreements with three key employees.
Recent Accounting Pronouncements
In March 2016, Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2016-06, Derivatives and Hedging (Topic 815) (ASU 2016-06). ASU 2016-06 clarifies the requirements for assessing whether contingent call or put options that can accelerate the payment of principal on debt instruments are clearly and closely related to their debt hosts. This guidance is effective for annual reporting periods beginning after December 15, 2016, including interim periods within those annual periods. Early application is permitted. The Company is currently evaluating the impact of this new standard.
In February 2016, FASB issued ASU 2016-02, Leases (Topic 842) (ASU 2016-02). ASU 2016-02 will require lessees to recognize a lease liability and a right-of-use asset for all leases, with the exception of short-term leases, at the commencement date. This guidance is effective for annual reporting periods beginning after December 15, 2018, including interim periods within those annual periods. Early application is permitted. The Company is currently evaluating the impact of this new standard.
In November 2015, FASB issued ASU 2015-17, Income Taxes (Topic 740) (ASU 2015-17). ASU 2015-17 simplifies the presentation of deferred income taxes by requiring that deferred tax assets and liabilities be classified as non-current in a classified statement of financial position. This guidance is effective for annual reporting periods beginning after December 15, 2016, including interim periods within those annual periods, with early adoption permitted. The Company early adopted ASU 2015-17 for the year ended December 31, 2015 on a prospective basis, as permitted. There was no impact of early adoption of ASU 2015-17 on the Company’s consolidated financial statements previously reported.
In April 2015, FASB issued ASU 2015-03, Interest – Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs (ASU 2015-03). ASU 2015-03 requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. This guidance is effective for annual reporting periods beginning after December 15, 2015, and interim periods within fiscal years beginning after December 15, 2016, with early adoption permitted. The Company early adopted ASU 2015-03 in July 2015, as permitted. There was no impact of early adoption of ASU 2015-03 on the Company’s consolidated financial statements previously reported.
| 66 |
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements – Going Concern (Subtopic 205-40) (ASU 2014-15). ASU 2014-15 defines management’s responsibility to evaluate whether there is substantial doubt about an organization’s ability to continue as a going concern and provides guidance on the related footnote disclosures. This guidance is effective for annual reporting periods beginning after December 15, 2016, and interim periods within annual periods beginning after December 15, 2016. Early application is permitted. The Company is currently evaluating the impact of this new standard.
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers (Topic 606) (ASU 2014-09). ASU 2014-09 supersedes the revenue recognition requirements in Accounting Standards Codification, or ASC, Topic 605, Revenue Recognition, and most industry-specific guidance. The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods or services. In August 2015, the FASB issued ASU 2015-15, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date, which defers the effective date of ASU 2014-09 for all entities by one year. This guidance is currently effective for annual reporting periods beginning after December 15, 2017, including interim periods within that reporting period, under either full or modified retrospective approach. Early application is permitted as of annual reporting periods beginning after December 15, 2016. The Company is currently evaluating the impact of this new standard on its revenue recognition policy.
The Company has considered other recent accounting pronouncements and concluded that they are either not applicable to the business or that no material effect is expected on the consolidated financial statements as a result of future adoption.
Available Information
Our website address is www.xeneticbio.com. The information in, or that can be accessed through, our website is not part of this Registration Statement on Form S-1. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K and amendments to those reports are available, free of charge, on or through our website as soon as practicable after we electronically file such forms, or furnish them to, the U.S. Securities and Exchange Commission (SEC). The public may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Information on the operations of the Public Reference Room can be obtained by calling 1-800-SEC-0330. The SEC maintains an internet site that contains reports, proxy and information statements and other information regarding our filings at www.sec.gov.
In addition to disclosing current information pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 and for reports of information required to be disclosed by Regulation FD through our SEC filings, we also intend to disclose such current information through our investor relations website, press releases, public conference calls, webcasts and through various social media channels, including Facebook, Twitter, LinkedIn, Google+ and Chairman’s Blog Profile.
We have considered other recent accounting pronouncements and determined that they are either not applicable to our business or that no material effect is expected on the consolidated financial statements as a result of future adoption.
| 67 |
JOBS Act
Under Section 107(b) of the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, an “emerging growth company” can delay the adoption of new or revised accounting standards until such time as those standards would apply to private companies. We have irrevocably elected not to avail ourselves of this exemption and, as a result, we will adopt new or revised accounting standards at the same time as other public companies that are not emerging growth companies. There are other exemptions and reduced reporting requirements provided by the JOBS Act that we are currently evaluating. For example, as an emerging growth company, we are exempt from Sections 14A(a) and (b) of the Exchange Act which would otherwise require us to (i) submit certain executive compensation matters to stockholder advisory votes, such as “say-on-pay,” “say-on-frequency” and “golden parachutes” and (ii) disclose certain executive compensation related items such as the correlation between executive compensation and performance and comparisons of our Chief Executive Officer’s compensation to our median employee compensation. We also intend to rely on certain other exemptions, which include but are not limited to, providing an auditor’s attestation report on our internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and complying with any requirement that may be adopted by the PCAOB regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis.
We will remain an “emerging growth company” until the earliest of the following: the last day of the fiscal year following the fifth anniversary of the date of the completion of the Company’s first offering, which was declared effective by the Commission on March 22, 2012; the last day of the fiscal year in which our total annual gross revenues are equal to or more than $1 billion; the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
| 68 |
Business
Overview
We are a clinical-stage biopharmaceutical company focused on discovery, research and development of next-generation biologic drugs and novel orphan oncology therapeutics that may contribute to improvements in global human health. Our 200+ patent portfolio covers next generation biologic drugs and novel oncology therapeutics and provides protection for our current drug candidates and positions as well as strategic partnership and commercialization opportunities.
Our objective is to leverage our portfolio to maximize out-license opportunities that generate working capital to both build incremental shareholder value and provide funding necessary to clinically develop our orphan oncology drug candidate pipeline through to market launch.
We incorporate our patented and proprietary technologies into a number of drug candidates currently under development either in-house or with biotechnology and pharmaceutical collaborators in order to create what we believe will be the next-generation biologic drugs and therapeutics. While we primarily focus on researching and developing orphan oncology drugs, we also have significant interests in drugs being developed by our collaborators to treat, among others, hemophilia and anemia. Among others, we are working together with Shire to develop a novel series of polysialylated blood coagulation factors, including a next generation BAX 826. This collaboration relies on our PolyXen technology to conjugate PSA to therapeutic blood-clotting factors, with the goal of improving the pharmacokinetic profile and extending the active life of these biologic molecules. Shire is one of our largest shareholders having invested $10M in our common stock during 2014. The agreement is an exclusive research, development and license agreement which grants Shire a worldwide, exclusive, royalty-bearing license to our PSA patented and proprietary technology in combination with Shire’s proprietary molecules designed for the treatment of blood and bleeding disorders. Under the agreement, we may receive regulatory and sales target payments for total potential milestone receipts of up to $100 million plus royalties on sales.
Our four core proprietary technologies are: PolyXen, OncoHist, Virexxa and ImuXen. These four proprietary technologies may be used in a variety of drug candidates providing potential use advantages over competing products. Our and our collaborators’ pipeline altogether consists of three (3) drug candidates using the PolyXen platform delivery technology for four (4) different indications, the OncoHist molecule for two (2) indications, and the small molecule Virexxa for two (2) indications. All of our drug candidates are in the development stage and none has yet received regulatory approval for marketing in the U.S. by the FDA or by any applicable agencies in other countries. Our lead product candidates include ErepoXen, a polysialylated form of erythropoietin (EPO) for the treatment of anemia in pre-dialysis patients with chronic kidney disease, and FDA Orphan Drug designated oncology therapeutics Virexxa and OncoHist for the treatment of progesterone receptor negative endometrial cancer and refractory acute myeloid leukemia, respectively.
We, directly or indirectly, through our wholly-owned subsidiary, Xenetic U.K., and its wholly-owned subsidiaries, Lipoxen, Xenetic Technologies, Inc. and SymbioTech, GmbH, own various U.S. federal trademark registrations and applications, and unregistered trademarks and servicemarks, including but not limited to Virexxa, OncoHist, PolyXen, ErepoXen, ImuXen, Xemys, and PulmoXen. Altogether, we, directly or indirectly, hold more than 201 patents with 40 in the United States and an additional 161 international patents, and we have approximately 101 pending patent applications worldwide.
Our Company Origins, Team and Investors
The Company was formerly known as General Sales and Leasing, Inc. before it changed its name to Xenetic Biosciences, Inc. in January 2014. On January 23, 2014, the Company consummated an acquisition pursuant to a written plan of reorganization, in which it performed a reverse merger with Xenetic Biosciences Limited (UK) (formerly Xenetic Biosciences PLC), or Xenetic UK, a company incorporated in England and Wales under the Companies Act of 1985 and for a period of time tracked on the London AIM exchange. Xenetic Biosciences PLC had been formed in 1997 as a spin out from The School of Pharmacy, University of London, and was based in London, England, until the end of 2013. Upon the reverse merger Xenetic UK became a wholly owned subsidiary of the Company. Specifically, upon completion of the reverse merger, the Company acquired all issued and outstanding shares of capital stock of Xenetic UK. At that time, because former Xenetic UK shareholders owned approximately 97% of the combined company on a fully diluted basis and all members of the combined company’s executive management were from Xenetic UK, Xenetic UK was deemed to be the acquiring company for accounting purposes and the transaction was accounted for as a reverse acquisition in accordance with accounting principles generally accepted in the United States, or U.S. GAAP.
| 69 |
Our move from England to Lexington, Massachusetts, together with coordinating our R&D effort through our new U.S. Lexington facility caused us to close our laboratory facilities in the U.K.
We have attracted significant funding and prominent investors from leading healthcare companies, including Shire, SynBio, Serum Institute and OPKO Health Inc., a NYSE listed pharmaceutical company.
Our Strategy
Our goal is to become a leader in the development of novel orphan oncology drugs while leveraging our proprietary delivery technology as a vehicle for creating next generation bio-therapeutics.
Our strategy is to pursue a continuous and ongoing effort of out-licensing our PolyXen platform technology to drive short-term, incremental shareholder value and generate working capital to assist in providing the funding required to support our long-term development of orphan oncology drug candidates through regulatory approval and commercialization.
We advance our PolyXen platform technology through collaborative out-license arrangements with global pharmaceutical companies that can apply the resources necessary to bring the drug candidate to worldwide commercialization and with other partners that in-license our technology on a restrictive-market basis. The latter provides access to clinical data which can assist us in making decisions about potential monetization in larger markets.
We believe our Orphan Drug oncology candidates may meet an established and unmet therapeutic need for a relatively limited population of patients, and products with very high sales potential – benefiting from more favorable price and reimbursement policies.
We advance our drug candidates through a combination of conducting our own in-house research and through the use of contract manufacturing and contract research organizations in order to efficiently manage the Company’s overheads. Continuous pipeline growth and advancement of out-licensed drug candidates is dependent, in part, on several important co-development collaborations and strategic arrangements. Together with our collaborative partners, we are focused on developing our pipeline of next generation bio-therapeutics and novel orphan drugs in oncology based primarily on our PolyXen, OncoHist and Virexxa proprietary technologies.
Ultimately, our collaborative out-licensing agreements relating to the technologies are an integral part of our early-stage monetization strategy, and we are substantially dependent on several important collaborations and strategic arrangements with Shire, Serum Institute, SynBio and Pharmsynthez, an affiliate of our controlling shareholder SynBio. If successful, our collaborative strategy could:
| · | facilitate a global market launch of our drug candidates; | |
| · | facilitate the monetization of our investment to date in the drug candidates by way of an upfront license payment and milestone payments as a product is advanced through the clinic trials; | |
| · | expedite any royalty revenues if the drug candidate is taken to market by an already leading provider with an established market presence; | |
| · | decrease demands on our own financial and working capital resources; | |
| · | provide for reallocation of resources to provide for reallocation of the in-house development and marketing of new orphan candidates; and | |
| · | allow better financial and clinical control throughout the process from pre-clinical development, through IND application, human clinical trials, and potentially market approval and product launch. |
Specifically, for the Company’s PolyXen based next generation biologics vested in our pipeline via its various collaborations (e.g., ErepoXen), we will develop to a stage that will enable us to seek profitable out-licensing arrangements with major pharmaceutical companies for further development and eventual commercialization, in exchange for milestone payments and royalties from product sales. We are also pursuing out-licensing of PolyXen for use with other molecules of our collaborators’, similar to our arrangement with Shire, in exchange for upfront payments, clinical milestones and royalties linked to sales. In addition, we plan to develop next generation biologics utilizing PolyXen (as exemplified by ErepoXen) to a clinical stage that will enable us to seek profitable out-licensing arrangements, which we anticipate will include milestone payments and royalties from product sales.
| 70 |
While we advance our drug candidates by combining our in-house research with that of outside resources in order to efficiently manage our overhead, we will still need to raise additional working capital to develop our drug candidates to the point of commercialization. Although we are optimistic, there can be no assurance that we will be successful in raising such additional capital. If not successful, our business could be adversely affected, and potentially reduce your overall return and dilute the value of your investment in shares of our common stock.
In-House Research, Outside Services and Collaborations
We are focused on developing our pipeline of next generation bio-therapeutics and novel orphan oncology drugs based on our PolyXen, OncoHist and Virexxa proprietary technologies. In order to do this while efficiently managing our overhead, we rely on in-house research, services of contract manufacturers and contract research organizations and strategic collaborations. As such, continuous pipeline growth and advancement of technologies and drug candidates is dependent, in part, on several important collaborations and strategic arrangements with, among others:
| · | Shire, a global biopharmaceutical leader and significant shareholder of ours; | |
| · | SynBio, a Russian pharmaceutical company and significant shareholder of ours; | |
| · | Pharmsynthez, a Russian pharmaceutical company and significant shareholder of ours; and | |
| · | Serum Institute, one of the world’s largest vaccine manufacturers and India’s largest biotech companies and significant shareholder of ours. |
Accordingly, in addition to our pipeline of next generation bio-therapeutics and novel orphan oncology drugs, we also have significant interests in drugs being developed by our collaborators to treat, among others, hemophilia and anemia, where we expect to collect milestone royalties. For further detail, please read the section named “Significant Co-Development Collaborations and Strategic Arrangements” on page 74.
Product Pipeline and Market Opportunities
The Technologies
We incorporate our patented and proprietary technologies into a number of drug candidates currently under development either in-house or with biotechnology and pharmaceutical collaborators in order to create what we believe will be the next-generation biologic drugs and therapeutics. While we primarily focus on researching and developing orphan oncology drugs, we also have significant interests in drugs being developed by our collaborators to treat, among others, hemophilia and anemia. Our four core proprietary technologies are:
| PolyXen | An enabling biological platform technology designed to extend the circulation in the human body for a variety of existing drug molecules and, thereby, to create potentially superior next generation drug candidates. PolyXen is based on the concept of polysialylation and utilizes polysialic acid, or PSA, which is a biopolymer, comprising a chain of sialic acid molecules. PSA is a natural constituent of the human body, though we obtain our PSA from a bacterial source. |
| Virexxa | A small molecule therapeutic with the potential to confer sensitivity to cancer cells to hormone therapeutics that are otherwise insensitive to such treatments. Virexxa, sodium cridanimod, belongs to a class of low-molecular weight synthetic interferon inducers. In addition to its immunomodulatory properties, Virexxa has been shown to increase levels of progesterone receptor expression in tumor tissue of patients who are progesterone receptor deficient, and thus may restore sensitivity of non-responsive endometrial cancers to hormonal (e.g., progestin) therapy. Based on preclinical observations, Virexxa may also be therapeutically relevant in other hormone-resistant cancers, such as triple-negative breast cancer. Virexxa has been granted an Orphan Drug Designation by the FDA, for treatment of progesterone receptor negative endometrial cancer in conjunction with progesterone therapy. |
| OncoHist | A novel therapeutic platform technology that utilizes the properties of modified human histone H1.3 for targeted cell necrosis or apoptosis programed cell death, which may enable OncoHist to treat a broad range of cancer indications. OncoHist, unlike many competing oncology therapies, is based on a molecule occurring naturally in the human body, in the cell nucleus, and is therefore expected to be less toxic and immunogenic than other oncology therapies. |
| ImuXen | A novel liposomal co-entrapment encapsulation technology designed to maximize both cell and immune system mediated responses. The technology is based on the co-entrapment of the nominated antigen(s) in a liposomal vesicle. The technology when applied may create new vaccines and improve the use and efficacy of certain existing human vaccines. |
| 71 |
Our four proprietary technologies may be used in a variety of drug candidates providing potential use advantages over competing products. Our and our collaborators’ pipeline altogether consists of three (3) drug candidates using the PolyXen platform delivery technology for four (4) different indications, the OncoHist molecule for two (2) indications, and the small molecule Virexxa for two (2) indication. All of our drug candidates are in the development stage and none has yet received regulatory approval for marketing in the U.S. by the FDA or by any applicable agencies in other countries.
PolyXen
PolyXen is a drug delivery technology designed to extend the circulation in the human body for a variety of existing drug molecules and, thereby, to create potentially superior next generation drug candidates. It is based on the concept of polysialylation and utilizes polysialic acid (PSA), which is a biopolymer, comprising a chain of sialic acid molecules. PSA is a natural constituent of the human body, though Xenetic obtains its PSA from a bacterial source.
Sialic acid is found on the external membrane of a number of cell types in the body. In addition, it is a natural component expressed on the external membrane on a number of bacterial types. The chain of sialic acid molecules can be anywhere from 4 to over 200 individual sialic acid molecules in length. We use the linear form of PSA called colominic acid. It is a natural, hydrophilic polymer isolated from a bacterial strain of E. coli K1. This natural glycan is negatively charged, non-toxic and is biodegradable. The PSA chain is extensively purified from large-scale bacterial cultures under current Good Manufacturing Practices (cGMP), modified to specified sizes and then attached to defined sites on the therapeutic in order to enhance the properties of the therapeutic. The major effect of PSA addition to a therapeutic is to change the apparent hydrodynamic radius of the molecule. This physical alteration then changes a number of the biological characteristics of the therapeutic.
Both the length and the site of attachment of the PSA chain can enhance the properties of the therapeutic. The most noticeable, and perhaps the most relevant enhancement, is an extension of the lifetime of the therapeutic in blood circulation. This is due to the increase in the size of the drug which results in a decrease in the clearance rate of the molecule in the kidney by glomerular filtration. In addition, studies have shown that using PolyXen changes in other biological characteristics of the therapeutic such as protease sensitivity and temperature sensitivity. The linked PSA molecules may be less viscous in solution compared to other technologies, potentially providing for easier injections and fewer adverse injection site reactions.
The current standard for certain biologic delivery agents is methyl polyethylene glycol (or PEG) which is attached similarly to therapeutics. The mode of action between PSA and PEG is similar, increasing the apparent size of the molecule and thereby increasing the circulating time of the drug in the blood. PEGylation is a proven technology that can offer advantages in terms of pharmacokinetics and pharmacodynamics for therapeutics over non-modified, first generation molecules. There are a number of PEG modified molecules on the market, in clinical trials and under development. However, PEGylation is deemed to have limitations. It is not biodegradable and may therefore at high doses result in intra-cellular accumulation, potentially leading to vacuole formation in the cells. In comparison, PSA is a chain of sialic acids which is a natural constituent of the human body. PSA is biodegradable into individual sialic acid units. PEG has also been shown to be immunogenic when coupled to proteins and can activate the complement system showing limitations on particular molecules. PSA has to date been shown to be non-immunogenic as well as demonstrating greater versatility and fewer limitations on early-stage development relative to PEG. We believe PSA may provide the advantages of PEG, which represents a multi-billion dollar market, without its disadvantages, offering a potential advance over PEG molecules.
We plan to develop drug candidates, such as ErepoXen that uses the PolyXen platform delivery technology, to a stage that will enable us to seek profitable out-licensing arrangements with major pharmaceutical companies for further development and eventual commercialization, in exchange for milestone payments and royalties from product sales. We are also pursuing outlicensing PolyXen for use with a partner’s proprietary molecule in exchange for upfront payments, clinical milestones and royalties linked to sales. In general, our collaborative out-licensing agreements relating to the platforms are an integral part of our early-stage monetization strategy.
In addition to potentially enhancing therapeutics, we believe that adding PSA to an existing marketed biological drug may allow for patent extension, thereby potentially creating a patent-protected next generation candidate. We are exploring such opportunities.
| 72 |
Virexxa
On November 13, 2015, we entered into the Kevelt APA with Kevelt, and Pharmsynthez. Pursuant to the Kevelt APA, Kevelt and Pharmsynthez, transferred to us certain intellectual property rights with respect to Virexxa, and the worldwide rights to develop, market and license Virexxa for certain uses, except for excluded uses within the CIS in exchange for, among others, 3,378,788 shares of our common stock. Virexxa is a Phase II oncology drug candidate which is under investigation for the treatment of certain endometrial cancers. As part of this total consideration, we also acquired Kevelt's U.S. Orphan Drug designation for the use of Virexxa in the treatment of progesterone receptor negative endometrial cancer in conjunction with progesterone therapy.
Virexxa is our most advanced candidate with an orphan designation for a subset of endometrial cancers and an IND in effect for Phase II clinical trials in the U.S. and certain territories in Eastern Europe. While Virexxa (a sodium cridanimod), belongs to a class of low-molecular weight synthetic interferon, or IFN, inducers and is primarily used in a wide range of therapeutic areas such as antiviral, antibacterial, antitumor, and inflammatory indications due to its ability to modify or regulate one or more immune system functions, Virexxa may prove to be therapeutically relevant in hormone-resistant cancers by increasing the levels of progesterone receptor, or PR, expression in tumor tissue of patients who are PR deficient. As such, it may restore the sensitivity of non-responsive endometrial cancers to hormonal (e.g., progestin) therapy. Accordingly, we are pursuing the use of Virexxa for endometrial cancer.
OncoHist
OncoHist is a therapeutic platform technology that utilizes the recombinant, modified, properties of the human histone H1.3 (H1.3) for targeted cell killing by cell necrosis or apoptosis-programmed cell death.
OncoHist may be a drug candidate to treat a broad range of cancer indications. It, unlike many competing oncology therapies, is based on a molecule occurring naturally in the human body, in the cell nucleus, and is therefore expected to be less toxic and immunogenic than other oncology therapies. We developed a novel form of the H1 histone molecule and were granted patent protection of the new chemical entity, N-bis-met-histone 1.3, or OncoHist, in use against cancer through at least 2027.
OncoHist is based on research covered under our patent portfolio related to novel functions of histones. Histone H1 has strong anti-proliferative properties against cancer cells of different histological origin. This has been demonstrated extensively for hematologic malignancies, such as leukemias, lymphomas, and myelomas, and also for tumors from other tissues. Susceptibility of cells to the cytotoxic effect of histones is determined by the ability of histone H1 to selectively destabilize the tumor cell membrane, which results in cell death. OncoHist was tested on 58 tumor cell lines derived from various tissues. Hematopoietic cancer cell lines were found to be among the cell lines the most sensitive to OncoHist. OncoHist binds to the cell membrane and the binding mechanism appears to be completely different from that of other therapeutic agents on the market for hematopoietic cancers. The Dana-Faber Cancer Institute is currently conducting additional studies of the OncoHist binding mechanism. Hematopoietic cancer lines resistant to current chemotherapeutic agents have still been sensitive to OncoHist.
We plan to develop drug candidates such as OncoHist for AML, which uses the OncoHist platform technology, to a stage that will enable us to seek profitable outlicensing arrangements or commercialization. A Phase I/II trial to evaluate the safety and tolerability of OncoHist alone for AML was conducted in 2004-2007 at Saarland University, in Germany with 22 AML patients. Formal criteria of response were not met in any of the patients. However, according to the overall assessment of the investigator three patients achieved a partial remission although the strict criteria for partial remission according to protocol were only partly fulfilled. Six patients had a temporary increase of their platelet count while on therapy during the follow-up period. Most notably, two patients who had received two treatment cycles each experienced stabilization of their disease for six and one-half and 16 months, respectively.
| 73 |
ImuXen
ImuXen is a patented platform technology based on the concept of simultaneous delivery of multiple active pharmaceutical ingredients (APIs), as antigens with the same liposome. The liposomes are composed of lipids that encapsulate an aqueous core. The APIs can be trapped in the core, be associated with the lipids, or both. Proteins, peptides, nucleic acids, polysaccharides and live or inactivated infectious agents can all be used as an API with the same liposome. Both the size and the lipid composition can be controlled which affects the biological properties of the liposome. Manufacturing involves the passive entrapment of the vaccine APIs by freeze drying commercially available liposomes with the antigens of interest. When the material is rehydrated it yields liposomes with the entrapped APIs.
Having multiple APIs formulated with the same liposome allows simultaneous delivery of the antigens to the same antigen presenting cell. This may allow a more efficient immune response to all the agents presented. In addition, it is possible that multiple vaccines can be delivered with a single injection. Relevant pre-clinical studies have shown a reduction in the number of doses and dosage required, and a faster immune response time. This efficient immune response also may allow for use of antigens that traditionally give a poor antibody response.
This technology is not currently the focus of clinical development for the Company. However, through a license agreement with Pharmsynthez, there is a novel multiple sclerosis vaccine that is in clinical development in Russia. SynBio completed a Phase I/II clinical trial to treat relapsing remitting multiple sclerosis (RRMS), and secondary progressive multiple sclerosis (SPMS) in the CIS. Peptides corresponding to antigenic sections of basic myelin protein were encapsulated within liposomes to be used as the therapeutic agent in our drug candidate, Xemys, that uses the ImuXen platform technology. As an integral part of our strategy, we await later stage clinical data on Xemys to determine whether to progress this candidate into U.S. clinical trials for potential out-licensing.
Drug Candidates in the Pipeline
Our four proprietary technologies may be used in a variety of drug candidates providing potential use advantages over competing products. All of our drug candidates are in the development stage and none has yet received regulatory approval for marketing in the U.S. by the FDA or by any applicable agencies in other countries. The following table summarizes key information regarding the current pharmaceutical product candidates, organized by our corporate programs and our collaborators’ programs:
| Xenetic Corporate Programs | |||||||||
| Drug Candidate | Technology | Indication | ICH | Orphan Disease | Description, Status and Next Catalyst | Pre-clinical | Phase 1 | Phase 2 | Phase 3 |
| Virexxa | Virexxa | Endometrial Cancer | ✔ | ✔ | VIR-EC-01: Phase II trial in progress under a US IND (FDA Orphan Designation); Q4 2016 – expect US trial expanded protocol to commence | ||||
| ErepoXen | PolyXen | Anemia | ✔ | PSA-EPO-06: Phase II trial being conducted in Australia, South Africa and New Zealand. Cohort III in progress; Q3 2016 – expect report Phase 2 cohort 3 | |||||
| OncoHist | OncoHist | Acute Myeloid Leukemia | ✔ | ONC-AML-01: Pre-clinical studies and pre-IND meeting with the FDA is complete. Negotiations with contract manufacture and clinical research organizations are in progress (FDA Orphan Designation); H2 2017 – expect to file IND | |||||
| Virexxa | Virexxa | Triple Negative Breast Cancer | ✔ | VRX-BC-01: Pre-clinical studies under development; H2 2016 - expect to file IND | |||||
| Indicates programs completed. | ||
| Indicates programs in progress in such phase. |
| 74 |
| Collaborative Partner Programs (alphabetical by clinical developer) | ||||||||||
| Drug Candidate | Technology | Sponsor | Indication | ICH | Orphan Disease | Description and Status | Pre-clinical | Phase 1 | Phase 2 | Phase 3 |
| BAX 826 | PolyXen | Shire | Hemophilia | ✔ | ✔ | PSA-FVIII: CTA in the U.K. for a Phase I/II clinical trial was approved. Clinical trial in the U.K. commenced in Q1 2016. | ||||
| PulmoXen | PolyXen | Pharmsynthez | Cystic Fibrosis | ✔ | PMO-CF-01: Phase I completed in Russia. A Phase II clinical trial is expected to start Q4 2016 in Russia. | |||||
| Xemys | ImuXen | Pharmsynthez | Multiple Sclerosis | IMU-MS-01: Phase I dose ranging study in Russia is complete. | ||||||
| Oxyntolong | PolyXen | Pharmsynthez | Type II Diabetes and Obesity | OXN: Phase 1 completed in Russia. Phase 2 commenced in Russia. | ||||||
| ErepoXen | PolyXen | Serum Institute | Anemia | ✔ | PSA-EPO-03: Phase II(a) intravenous and subcutaneous human clinical trials conducted in India are complete. The study report is expected in Q2 2016. | |||||
| ErepoXen | PolyXen | SynBio | Anemia | PSA-EPO-05: Russian Phase II(b)/III in progress. | ||||||
| OncoHist | OncoHist | SynBio | AML | ✔ | Onc-AML-02: Russian Phase II is on hold pending protocol revision due to a change in Russian Standard of Care requirements. | |||||
| OncoHist | OncoHist | SynBio | NHL | Onc-NHL-01: Russian Phase II dose ranging studies are completed in Russia. | ||||||
| Indicates programs completed. | ||
| Indicates programs in progress in such phase |
| 75 |
Virexxa
General
Our decision to investigate Virexxa® for the treatment of endometrial cancer was based in part on the history of sodium cridanimod in preclinical and clinical research conducted by others, including 22 clinical trials conducted and completed in Russia by other clinical developers that assessed efficacy and safety of sodium cridanimod. Sodium cridanimod is approved for marketing in the Russian Federation, and numerous CIS countries for the treatment of certain infections, including for use in immunodeficient patients. Sodium cridanimod has been approved for medicinal use and marketed in the Russian Federation and former Soviet states for more than 20 years and more than eleven million doses have been sold for non-cancer indications. Virexxa® (sodium cridanimod), is also known under the brand names Neovir, Camedon and Primavir.
Endometrial cancer is the most common gynecological cancer in the U.S. with an estimated 60,000 new cases in 2016 of which twenty percent (20%) are recurrent disease. Ten percent (10%) of patients with endometrial cancer are PrR-negative or desensitized to progestin therapy with PrR tumors designated Stage III or IV and where surgical, radio- or chemo-therapies have failed or are not an option. For this patient group, there is a less than forty percent (40%) five-year survival rate and many die within one (1) year. Based on the current standard of care, the endometrial cancer market opportunity is estimated at approximately $450 to $650 million.
Virexxa has been granted an Orphan Drug Designation by the FDA for treatment of progesterone receptor negative endometrial cancer in conjunction with progesterone therapy. In model cases, Virexxa efficiently induces expression of both PrA and PrB and supports effective progestin therapy in PrR-negative endometrial cancer cases. Because of the extensive clinical testing of sodium cridanimod for immunomodulatory purposes indicating that sodium cridanimod is safe and well-tolerated as a drug, we were able to proceed directly to a Phase II clinical in the United States. This study is currently active under the same IND (through Pharmsynthez/Kevelt) in Belarus and Ukraine. Of six (6) evaluable patients, four (4) patients responded to Virexxa. Stable disease for at least six (6) weeks was observed in four (4) patients and one (1) patient continues to receive experimental treatment one (1) year after commencing treatment. One (1) patient with stable disease was withdrawn from the study.
VIR-EC-01: Xenetic Virexxa Clinical Trial
Our development program for Virexxa (sodium cridanimod) includes an ongoing Phase II study under our US IND for Virexxa in conjunction with progestin therapy in a population of subjects with progesterone receptor negative (PR) recurrent or persistent endometrial cancer. This study is also active under the same IND (through Pharmsynthez/Kevelt) in Belarus and Ukraine. It is an open-label, multicenter, single-arm Phase II study calling for a total of 58 subjects with documented evidence of PR endometrial cancer as determined by immunohistochemistry.
The primary objective of this study is to assess the antitumor activity of Virexxa in conjunction with progestin therapy as measured by objective response rate (partial/complete) in women with progesterone receptor negative recurrent or persistent endometrial carcinoma not amenable to surgical treatment, radiotherapy, or chemotherapy.
Secondary objectives of the study include: (a) to assess progression free survival, time to response, time to progression, duration of overall survival and Overall Disease Control Rate for subjects receiving Virexxa and progestin therapy; and (b) to evaluate the safety and tolerability of Virexxa in conjunction with progestin therapy, as measured by adverse events, laboratory safety parameters, and cardiac safety assessments.
Additional translational objectives include determining the efficacy of Virexxa in combination with progestin on PR levels in tumor tissue, to assess the association of change of PR levels with efficacy variables and to assess pharmacokinetic data for Virexxa and progestins after a single-dose and multiple-dose administration.
| 76 |
ErepoXen
General
ErepoXen polysialylated erythropoietin (PSA-EPO) is the second most advanced drug candidate in our clinical pipeline. ErepoXen uses our PolyXen technology in treating anemia in Chronic Kidney Disease (CKD) patients. It is designed to reduce the dosing frequency by extending circulation time of the therapeutic in the body. ErepoXen is a polysialylated form of recombinant erythropoietin (EPO), a hormone produced by the kidneys to maintain red blood cell production and prevent anemia. Chronic renal failure or chemotherapy can cause anemia. ErepoXen is designed and is being studied to reduce the required frequency of dosage and side effects and to be less immunogenic than existing treatments. Clinical results of ErepoXen suggest that the drug candidate can be administered once per month. In comparison, other EPOs, such as Aranesp from Amgen and Mircera from Roche, require dosing every 2-4 weeks, whereas ErepoXen is designed to be administered every 6-8 weeks. The EPO market is estimated to reach $11.9 billion by 2020, with the Stage III-IV segment, which we target, to reach approximately $1.2 billion, with an expected compounded annual growth of 9.7% from 2014 to 2020. ErepoXen is a potentially mainstream drug with a substantial global market if we are successful.
In addition to researching and developing ErepoXen ourselves, SynBio and Serum Institute have collaborative arrangements to develop and launch ErepoXen in limited markets pursuant to which we will collect royalties.
Serum Institute conducted Phase I and Phase II clinical trials in 95 human subjects. These safety trials, which had no significant adverse events, provided us with the data to commence a Phase II repeat dosing, ICH compliant clinical trial for ErepoXen in Australia, New Zealand and South Africa for chronic kidney disease patients not on dialysis. We anticipate conducting three cohorts, of which we have completed two cohorts and fully recruited the third cohort. Each cohort represents an increased dose of ErepoXen that is given on a repeat schedule until therapeutic levels of hemoglobin are achieved. Both completed cohorts had no drug-related significant adverse events. In order to accelerate and finalize the Phase II clinical trial, we opened a South African arm for the third cohort. Serum Institute indicated that it will use its data from the Phase II clinical trials and data generated from Xenetic’s Phase II trial to further progress clinical trials of ErepoXen for in-center dialysis patients in India. 130 human subjects have been treated with ErepoXen without significant adverse effect.
SynBio applied for and received regulatory approval for ErepoXen to enter Phase II(b)/III human clinical trials in Russia. ErepoXen is in a 150-patient Phase II(b)/III clinical trial in Russia to directly compare ErepoXen to Aranesp. SynBio indicated that it will commence commercialization and marketing stage of ErepoXen in the Russian and the CIS markets if approved in such market.
Outside of Russia, we are seeking an out-license arrangement for the continuing development of ErepoXen as either a Phase II(b) or Phase III candidate with a well-capitalized license partner with its own experience at taking drug candidates through the latter stages of human clinical trials and executing a global market launch. Our strategy for ErepoXen could:
| · | facilitate a global market launch of ErepoXen; | |
| · | facilitate the monetization of our investment in ErepoXen by way of an upfront license payment and milestone payments as it is advanced through clinic trials; | |
| · | expedite any royalty revenues if ErepoXen is taken to market by an already leading provider with an established market presence; | |
| · | decrease demands on our own financial and working capital resources; | |
| · | provide for reallocation of resources to provide for reallocation of the in-house development and marketing of new orphan candidates; and | |
| · | allow better financial and clinical control throughout the process from pre-clinical development, through IND application, human clinical trials, and potentially market approval and product launch. |
| 77 |
ErepoXen Clinical Trials
PSA-EPO-06: Xenetic ErepoXen Clinical Trial. This is a Phase II open label, multicenter, sequential multiple dose finding study for subcutaneously administered PSA-EPO in CKD patients not on dialysis and not receiving erythropoiesis stimulating agents. It is being conducted in Australia, South Africa and New Zealand. Patients with hemoglobin levels between 8 and 10 grams per deciliter (g/dL) are given the drug candidate once every two weeks. When the hemoglobin level increases to between 10 and 12 g/dL the patient is moved to once every four week administration. The endpoints are safety and tolerability to repeat ErepoXen administration and to determine the dose of PSA-EPO that will move the patient’s hemoglobin level into the 10 to 12 g/dL range. We have finished the first three cohorts of patients with the drug well tolerated and no warning signals for safety so far. An interim report is in progress and we expect to report the results from that in Q4 2016.
The costs for this trial are being borne by us. Costs will depend on how many cohorts are treated. Serum Institute manufactured the Clinical material. Novotech Pty Limited (Novotech), Australia, is running the clinical trial.
PSA-EPO-05: SynBio ErepoXen (Epolong) Clinical Trial. This is a Phase IIb/III open label, comparative, multicenter, randomized, parallel group study for subcutaneously administered PSA-EPO in CKD patients not on dialysis and not receiving erythropoiesis stimulating agents to assess the drug’s safety, efficiency and tolerability in correction of anemia and maintenance of hemoglobin levels. Patients will be compared to a control arm with Aranesp (darbepoetin alfa). SynBio has conducted the study in the Russian Federation with three groups. All three groups have to have an initial hemoglobin level of between 8 and 10 g/dL. Group 1 patients receive the comparative drug, Aranesp once every two weeks throughout the study period of 24 weeks. Group 2 patients receive PSA-EPO once every two weeks throughout the study period unless the hemoglobin level goes above 12 g/dL. Group 3 patients receive PSA-EPO every two weeks until their hemoglobin levels are between 10 and 12 g/dL. Group 3 patients then receive PSA-EPO every four weeks. The starting dose for both clinical drugs is the lowest dose level. Dose adjustment, either up or down depending on patient response, occurs every four weeks. The trial is currently in progress. The total cost for this clinical trial is being borne by SynBio. Serum Institute manufactured the clinical material. OCT-Clinical Trials (OCT), Russia, is running the clinical trial.
PSA-EPO-03: Serum Institute ErepoXen Clinical Trial. This was a Phase I/II open label, multi-center, single escalating dose finding study for intravenously administered PSA-EPO for CKD patients who are on dialysis. This intravenous trial followed the completion of two subcutaneous PSA-EPO clinical trials in India. The first was a Phase I single dose study for subcutaneously administered PSA-EPO in healthy volunteers. The second was a Phase II single dose range finding study for subcutaneously administered PSA-EPO in CKD patients not on dialysis. All trials were conducted in India. In the Phase I/II clinical trial, p atients with hemoglobin levels less than or equal to 11 g/dL were given a single dose of PSA-EPO. The patient’s pharmacodynamic, pharmacokinetic and immunogenic parameters were then followed for the next 28 days. Safety and experimental parameters was examined at the end of each dosing cohort before moving onto the next level. The trial has been finished. There were no Serious Adverse Events (SAEs), attributable to PSA-EPO reported in the cohort 1 analysis report. Analysis of the clinical data is in progress and a final report on the trial is expected in 2016. The total cost of the clinical trial is being borne by Serum Institute and the clinical material was manufactured by Serum Institute. The clinical trial is being run by SIRO Clinpharm Pvt. Limited of India.
OncoHist for AML
General
The next most advanced drug candidate is OncoHist, which utilizes the properties of modified human histone H1.3 for targeted cell killing. OncoHist for AML is for the treatment of relapsed or resistant acute myeloid leukemia (AML). We are currently developing OncoHist for AML and we anticipate filing an IND application for OncoHist for AML subject to funding. We anticipate to address unmet medical needs for the condition which has a low cure rate, and the current better drugs show only a thirty percent (30%) overall response rate. Based on the current standard of care, the AML U.S. and European market opportunity is estimated at approximately $600 million with a seven percent (7%) annual growth.
| 78 |
We have a sponsored research agreement with Dana Farber Cancer Institute intended to elucidate OncoHist’s mechanism of action as well as to characterize the responsiveness of various AML cell lines to OncoHist. Dr. Richard Stone, MD, Professor of Medicine at Harvard Medical School and Clinical Director of the Adult Leukemia Program at Dana-Farber Cancer Institute, presented data at the 2014 American Society of Hematology meeting (Blood, 2014 124(21):3604 “OncoHist, an rh Histone 1.3, Is Cytotoxic to Acute Myeloid Leukemia Cells and Results in Altered Downstream Signaling).
We completed non-clinical toxicity studies of OncoHist guided, in part, by clinical data supplied by SynBio and SymbioTec, GmbH. In August 2015, we had a productive, in-person pre-IND meeting with the FDA where manufacturing and clinical matters were addressed including guidance from the FDA regarding inclusion of an additional indication besides AML in our planned Phase I clinical trial. We completed the preclinical toxicity studies using, in part, clinical material supplied by SynBio. By using SynBio’s clinical data rather than developing our own data, we have reduced our research and development costs. We had a pre-IND meeting with the FDA regarding the drug substance and drug product acceptance criteria and the characterization plans of nonclinical and clinical products to initiate a Phase I/II clinical study. In addition, we plan to establish a second source to supply us with OncoHist™ material suitable for use in humans in a Phase I/II(a) clinical trial under cGMP. Plans to commence clinical trials in the U.S. have been delayed due to insufficient working capital necessary to establish a source of cGMP material suitable for these trials. We expect to commence Phase I-II(a) clinical trials for multiple indications in the U.S. towards the end of the second half of 2017.
OncoHist Clinical Trials
ONC-AML-01: Xenetic OncoHist Clinical Trial. We have completed preclinical toxicity studies using clinical material supplied by SynBio. We had a pre-IND meeting with the FDA. We expect to submit an IND filing for a Phase I/II clinical trial for AML to the FDA and commence clinical trials, but not before the end of 2017. We expect this to be an open label dose-findings study to assess the safety, tolerability and efficacy of OncoHist for adult patients with refractory or relapsed AML. This trial will be conducted in the U.S. Data from the previously completed work by Saarland University’s Phase I clinical trial and the SynBio clinical trials will be used to aid in the design of the clinical protocol. We expect the Phase I/II clinical trial material to be produced by a cGMP compliant manufacturing facility. Selection of the Clinical Research Organization (CRO) to run the trial is in progress.
The costs for the clinical trial are being borne by us. We will need to raise additional capital prior to commencing Phase I/II clinical trials.
ONC-AML-02: SynBio Arahist-09 Clinical Trial. This was a Phase I/II open label controlled randomized study to assess the safety, tolerability and efficacy of OncoHist in combination with cytarabine-based chemotherapy regimens (HAM) in adult patients with refractory or relapsed AML. This study was conducted in Russia. Patients in arm I (high dose chemotherapy) of the study are randomly assigned to three groups: Group A (OncoHist 628 mg/m2 ¸ HAM); Group B (OncoHist 10005 mg/m2 ¸ HAM); and Group C (HAM only). Patients of arm II (low dose chemotherapy) of the study are randomly assigned to two groups: Group D (OncoHist, 1005 mg/m2 ¸ LDAC); and Group E (LADC only). Patients in arm I (high dose chemotherapy) will receive one dose of therapy. Patients in arm II (low dose chemotherapy) will receive one or two cycles of the therapy, depending on its effects in the first cycle. The HAM regimen was based on the then current standard of care in Russia. The Russian Ministry of Health changed the standard of care, and the study is on hold. The total cost of the trial is borne by SynBio. The clinical material for this OncoHist trial was manufactured at the Shemyakin Institute in Moscow for SynBio. The trial was run by a partner-sponsored CRO in Russia.
ONC-NHL-01: SynBio Anahoret Clinical Trial. This was a Phase I/II multicenter dose escalation study to assess the safety, pharmacokinetics and efficacy of OncoHist in adult patients with refractory B-cell NHL. This study was conducted in Russia from 2011-2013. The trial is complete and the report submitted to the Russian MoH. It was shown that OncoHist was well tolerated in this trial. The total cost of the trial was borne by SynBio. The clinical OncoHist drug product was manufactured at the Shemyakin Institute in Moscow for SynBio. The trial was run by a partner-sponsored CRO in Russia.
If and when satisfactory clinical patient data comes out of this collaboration that provides the Company a level of comfort that the drug candidate is well-tolerated and effective, the Company may pursue its own development program for this candidate. However, the Company would have to raise additional capital to pursue its own development of this drug candidate.
| 79 |
PSA-FVIII: Shire BAX 826
PSA-recombinant BAX 826 or Factor VIII has been developed as a long acting therapeutic to treat hemophilia. BAX 826 uses the PolyXen platform technology to conjugate PSA to therapeutic blood-clotting factors, with the goal of improving the pharmacokinetic profile and extending the active life of these biologic molecules. Shire is running this program, which is in the clinical trial phase. Shire has agreed to meet strict due diligence time milestones based on: Clinical Trial Authorization submission in respect of Phase I/II clinical trials, Final Clinical Study Report Phase I/II and BLA submission all by fixed dates per the contract. The total cost of this program is being borne by Shire. There can be no assurance if or when Shire will actually achieve any of these due diligence milestones. Shire filed a CTA for the program in Q4 2015 and commenced human clinical trials during the first quarter of 2016. The stated goal of Shire is to have a significantly longer-acting FVIII, once weekly or less frequent infusions, to remain the world’s leader in Hemophilia therapies. Based on current standard of care, hemophilia currently is estimated to represent a $10 billion market opportunity and expected to reach approximately $13 billion by 2020 with an annual compounded growth rate of 4-6%.
Pipeline Expansion Opportunities
Operating under Xenetic licenses within their home markets, our collaborators generate pre-clinical and clinical data related to our technologies across a wide spectrum of therapeutic areas. Under these agreements, we retain all rights for major markets and co-owns the clinical data. We, therefore, have the opportunity to utilize the data in our decision making process regarding commercialization in major markets. For example, we expect to be able to utilize the results from substantially all of our clinical toxicity data and other clinical data generated in the development of OncoHist, Virexxa, ImuXen and PolyXen for a variety of orphan oncology indications and next generation biologic drugs.
For example, we believe we may be able to develop Virexxa for triple-negative breast cancer (TNBC) indications. Based on the current standard of care, the triple negative breast cancer market opportunity is estimated at approximately $470 million. Results from preclinical and exploratory studies conducted by a collaborative partner suggest that Virexxa can up-regulate (i.e., increase the levels of) estrogen receptor (ER) in certain tissue types. Proof of concept studies are being planned to investigate additional therapeutic opportunities for Virexxa in other hormone-resistant tumor types than endometrial cancer, including TNBCs.
We also believe that the nature of our technologies, including the PolyXen and ImuXen platforms, will allow us to pursue additional drug candidates for new indications based on existing and future scientific data. The following is a list of certain drug candidates we and our collaborators are working on:
PMO-CF-01: Pharmsynthez PulmoXen. Pharmsynthez completed in April 2014 a Phase I(a) open label two dose safety study for inhaled PSA-DNase 1 in cystic fibrosis (CF), patients in Russia. Six healthy volunteers per dose level inhaled a single dose of PulmoXen each day for seven days and then were examined for lung function and adverse events. Two dose levels were performed. No adverse events were reported and lung function was reported to be normal. Clinical trials with CF patients are in the start-up stage, with regulatory applications being prepared. The total cost of the trial is being borne by Pharmsynthez. The trial is being run by OCT, Russia, in Russia, Belarus and Ukraine.
If and when satisfactory clinical patient data comes out of this collaboration that provides us a level of comfort that the drug candidate is safe and efficacious, we will pursue our own development program for this candidate. However, we would have to raise additional capital to pursue its own development of this drug candidate.
IMU-MS-01: Pharmsynthez Xemys (Multiple Sclerosis). Pharmsynthez is conducting a Phase I open label clinical sequential dose finding study for subcutaneously administered Xemys (liposomes containing peptides for basic myelin protein) in healthy volunteers and patients. The study is being conducted in Russia. Six healthy volunteers were given a single subcutaneous low dose of Xemys. No safety concerns have been noted thus far. Nine Multiple Sclerosis (MS) patients were then given weekly, increasing doses of Xemys to identify the maximum tolerated dose. The dosing was completed and the patients are now being monitored. No safety concerns were identified thus far. An additional 12 patients will next be recruited for multiple doses at the maximum tolerated dose for the third stage of this clinical trial. The total cost for the clinical trial is being borne by Pharmsynthez. The clinical material was manufactured by Pharmsynthez. The clinical trial is being run by OCT of Russia.
If and when satisfactory clinical patient data comes out of this collaboration that provides us a level of comfort that the drug candidate is safe and efficacious, we will pursue our own development program for this candidate. However, we would have to raise additional capital to pursue our own development of this drug candidate.
| 80 |
Intellectual Property
We strive to protect and enhance the proprietary technology, inventions, and improvements that are commercially important to our business, including seeking, maintaining and defending patent rights, whether developed internally or licensed from our collaborators or other third-parties. Our policy is to seek to protect our proprietary position by, among other methods, filing patent applications in the United States and in jurisdictions outside of the United States covering our proprietary technology, inventions, improvements and product candidates that are important to the development and implementation of our business. We also rely on trade secrets and know-how relating to our proprietary technology and product candidates, continuing innovation, and in-licensing opportunities to develop, strengthen and maintain our proprietary position in the field of oncology. We also plan to rely on data exclusivity, market exclusivity, and patent term extensions when available. Our commercial success will depend in part on our ability to obtain and maintain patent and other proprietary protection for our technology, inventions, and improvements; to preserve the confidentiality of our trade secrets; to obtain and maintain licenses to use intellectual property owned by third-parties; to defend and enforce our proprietary rights, including any patents that we may own in the future; and to operate without infringing on the valid and enforceable patents and other proprietary rights of third-parties.
Our drug candidates are in various stages of development, each protected by patent and pending patent applications in the U.S. with the USPTO and in certain other developed countries. Generally, patents have a term of 20 years from the earliest priority date (subject to paying all maintenance fees when due). In some instances, patent terms can be increased or decreased, depending on the laws and regulations of the country or jurisdiction that issued the patent, through the filing of a provisional patent application or through such other mechanisms, such as patient term extension (PTE) or supplementary protection certificates (SPC). Our first issued patents are due to begin to expire starting in 2022 with the majority of the existing issued patents expiring between 2027 and 2030.
Our patent strategy is to file patent applications on innovations and improvements in those jurisdictions that comprise the major pharmaceutical markets in the world or locations where a pharmaceutical may be manufactured. These jurisdictions include, but are not limited to the U.S., U.K., Australia, Japan, Canada, South Korea, China, Hong Kong, India, Russia and certain other countries in the European Union (E.U.) and Asia, though we do not necessarily file a patent application in each of these jurisdictions for every patent family.
As of September 21, 2016, we, directly or indirectly through our wholly-owned subsidiary, Xenetic U.K., and its wholly-owned subsidiaries, Lipoxen, Xenetic Technologies, Inc. and SymbioTech, GmbH, own more than 200 U.S. and international patents and approximately 100 pending patent applications that cover various aspects of our technologies. We have filed patent applications, and plan to file additional patent applications, covering various aspects of our PolyXen platform technology covering polysialylation and advanced polymer conjugate technologies, as well as our proprietary product candidates, including ErepoXen and PulmoXen. More specifically, our patents and patent applications cover polymer architecture, drug conjugates, formulations, methods of manufacturing polymers and polymer conjugates and methods of administering polymer conjugates. We will also be filing additional patent applications where possible for Virexxa and OncoHist for additional indications.
Our patent portfolio contains patents and patent applications that encompass our OncoHist platform technology including use of histones for the treatment of different cancers. The OncoHist patent portfolio, acquired as part of our acquisition of SymbioTec GmbH in January 2012, includes OncoHist, a bis-Met histone. In addition, our licensed patent portfolio includes patents issued in jurisdictions outside of the United States and licensed patent applications pending in jurisdictions outside of the United States that are foreign counterparts to one or more of the foregoing U.S. patents and patent applications. The OncoHist portfolio also includes patents that cover the use of a histone protein as an antibiotic and to threat thrombocytopenia and further as an antimicrobial component of a personal care product.
We have received patent protection for certain therapeutics that use our PolyXen technology linking the specific therapeutic to a polysialic acid (PSA). These include PSA-erythropoietin (EPO), PSA-insulin and PSA-insulin like protein, BAX 826 (FVIII), PSA-DNAse I and PSA-granulocyte colony stimulating factor (GCSF). Further patents cover methods to prepare proteins that are linked to a polysialic acid (PSA). These method patents include those that link a PSA to a protein in a high pH solution as well as patents that use a process for producing an aldehyde derivative of a sialic acid through the opening and oxidation of a sialic acid unit. For instance, we have patent protection for a PSA linkage that can be at the N-terminus.
| 81 |
We have received patent protection for the production of PSA and the removal of endotoxin during the purification process. The removal of endotoxin occurs through the addition of a high pH solution to the PSA and a process to separate a polydisperse ionically charged polysaccharide, such as PSA, into fractions of different average molecular weight. This is accomplished through the use of a column and elution buffers with different and constant ionic strength and pH, resulting in a fractionated polysaccharide that has a molecular weight polydispersity of 1.1 or lower.
We also have in-licensed patents and patent applications directed to certain of our product candidates and related uses thereof. We also possess and in-license substantial know-how and trade secrets relating to the development and commercialization of our product candidates. Among others, we have in-licensed a patent portfolio from Ploughshare, a licensing arm of the United Kingdom Ministry of Defence. The patent portfolio covers a method used to entrap a water-soluble drug within a liposome when the drug is mixed. This patent portfolio compliments our own liposome patents and pending patent applications for certain uses of liposomes. Together, the in-licenses patents and our liposome patents are being applied in our cystic fibrosis (CF) products.
Issued patents can provide protection for varying periods of time, depending upon the date of filing of the patent application, the date of patent issuance, and the legal term of patents in the countries in which they are obtained. In general, patents issued for applications filed in the United States can provide exclusionary rights for 20 years from the earliest effective filing date. In addition, in certain instances, the term of an issued United States patent that covers or claims an FDA approved product can be extended to recapture a portion of the term effectively lost as a result of the FDA regulatory review period, which is called patent term extension. The restoration period cannot be longer than five years and the total patent term, including the restoration period, must not exceed 14 years following FDA approval. The term of patents outside of the United States varies in accordance with the laws of the foreign jurisdiction, but typically is also 20 years from the earliest effective filing date. However, the actual protection afforded by a patent varies on a product-by-product basis, from country-to-country, and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country, and the validity and enforceability of the patent.
In certain situations where we work with drugs covered by one or more patents, our ability to develop and commercialize our technologies may be affected by limitations in our access to these proprietary drugs. Even if we believe we are free to work with a proprietary drug, we cannot guarantee that we will not be accused of, or be determined to be, infringing a third-party’s rights and be prohibited from working with the drug or found liable for damages. Any such restriction on access or liability for damages would have a material adverse effect on our business, results of operations and financial condition.
The patent positions of pharmaceutical and biotechnology companies, such as ours, are uncertain and involve complex legal and factual issues. There can be no assurance that patents that have issued will be held valid and enforceable in a court of law. Even for patents that are held valid and enforceable, the legal process associated with obtaining such a judgment is time consuming and costly. Additionally, issued patents can be subject to opposition or other proceedings that can result in the revocation of the patent or maintenance of the patent in amended form (and potentially in a form that renders the patent without commercially relevant and/or broad coverage). Further, our competitors may be able to circumvent and otherwise design around our patents. Even if a patent is issued and enforceable, because development and commercialization of pharmaceutical products can be subject to substantial delays, patents may expire early and provide only a short period of protection, if any, following the commercialization of a products encompassed by our patent(s). We may have to participate in interference proceedings declared by the U.S. Patent and Trademark Office, which could result in a loss of the patent and/or substantial cost to us. Further, we understand that if any of our pending patent applications do not issue, or are deemed invalid following issuance, we may lose valuable IP protection.
U.S. and foreign patent rights and other proprietary rights exist that are owned by third-parties and relate to pharmaceutical compositions and reagents, medical devices and equipment and methods for preparation, packaging and delivery of pharmaceutical compositions. We cannot predict with any certainty which, if any, of these rights will be considered relevant to our technology by authorities in the various jurisdictions where such rights exist, nor can we predict with certainty which, if any, of these rights will or may be asserted against us by third-parties. We could incur substantial costs in defending ourselves and our partners against any such claims. Furthermore, parties making such claims may be able to obtain injunctive or other equitable relief, which could effectively block our ability to develop or commercialize some or all of our products in the U.S. and in other countries and could result in the award of substantial damages. In the event of a claim of infringement, we or our partners may be required to obtain one or more licenses from third-parties. There can be no assurance that we can obtain a license to any technology that we determine we require on reasonable terms, if at all, or that we could develop or otherwise obtain alternative technology. The failure to obtain licenses, if required, may have a material adverse effect on our business, results of operations and financial condition. Further, we may not be able to obtain IP licenses related to the development of our drug candidates on a commercially reasonable basis, if at all.
| 82 |
It is our policy to require our employees and consultants, outside scientific collaborators, sponsored researchers and other advisors who receive confidential information from us to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third-parties except in specific circumstances. The agreements provide that all inventions conceived by an employee shall be our property. There can be no assurance, however, that these agreements will provide meaningful protection or adequate remedies for our trade secrets in the event of unauthorized use or disclosure of such information.
Significant Co-Development Collaborations and Strategic Arrangements
Shire plc
In August 2005, we entered into an exclusive research, development, license and supply agreement with what is now Shire plc (initially entered into with Baxter SA and Baxter Healthcare Corporation, and subsequently transferred to Baxalta which was acquired by Shire) to develop products with an extended half-life of certain proteins and molecules using our patent protected PolyXen technology whereby polysialic acid (PSA” – a chain of polysialic acids) is conjugated with Baxter’s proprietary molecule(s) designed to create a longer-acting hemophilia drug, a polysialylated recombinant BAX 826 (rFVIII) protein than what is currently available on the market. Baxter also has rights that extend to treatments of the failure of blood to coagulate. Baxter commenced human clinical trials on this novel drug candidate during the first quarter of 2016.
During June 2015, in connection with the separation of its biopharmaceuticals business to form Baxalta Incorporated, Baxter assigned all of its rights and obligations under its existing agreement with us to Baxalta, which was acquired by Shire.
This agreement has been amended several times since 2005, most recently in January 2014. The January 2014 amendment provides for increased future development, regulatory, sales and deadline extension receipts, restructured target deadlines and royalty receipts on potential net sales. We are entitled to up to $100 million in potential development, regulatory, sales and deadline extension receipts, which are contingent on the performance of Shire achieving certain milestones. We are also entitled to scaled royalties on net sales.
In connection with this amendment, Shire also made a $10 million equity investment in us at a price of $30.86 per share, which is a post money market cap of approximately $140 million in exchange for 324,097 shares of our common stock during January 2014.
Through December 31, 2015, we, together with Shire, continued to engage in research and development activities. No amounts were recognized as revenue during the six months ended June 30, 2016 or 2015, nor the years ended December 31, 2015 and 2014. Since August 2005, we have received approximately $19 million from Shire that includes milestone receipts, fees for services and a $10 million purchase of common stock of the Company in January 2014. We received a non-refundable $2 million payment from Shire in 2010 and granted Shire warrants to purchase approximately 4.6 million new shares of our common stock in connection with the 2010 amendment to the Baxter Agreement.
Shire has agreed to meet a number of clinical milestones with strict timelines under the 2014 amendment relating to: Clinical Trial Authorization (CTA) submission, Final Clinical Study Report and Biologics License Application (BLA) submission. Shire submitted a CTA application to the U.K. Medicines and Healthcare Products Regulatory Agency in late 2015 and commenced human clinical trials during the first quarter of 2016 in connection with this collaboration. There are very limited provisions to further modify the Baxter Agreement. There can be no assurance if or when Shire will actually achieve any of the remaining due diligence milestones. Among others, Shire may terminate the agreement without cause at any point following the so-called research midpoint or upon 90 days’ written notice. Further, the parties may mutually terminate the agreement upon material failure to comply with the material terms of the agreement or upon the other party’s insolvency.
Shire is a related party of ours, with a share ownership of approximately 4.8% of the total issued common stock as of October 20, 2016.
| 83 |
SynBio LLC
In August 2011, we entered into a stock subscription and collaborative development agreement with SynBio (Co-Development Agreement), pursuant to which we granted SynBio an exclusive license to develop, market and commercialize certain drug candidates utilizing molecules based on our PolyXen and OncoHist platform technologies in Russian and the CIS, (including Armenia, Azerbaijan, Belarus, Kazakhstan, Kyrgyzstan, Republic of Moldova, Tajikistan, Turkmenistan and Uzbekistan), collectively referenced to herein as SynBio Market. In exchange for our granting to SynBio those certain license rights, SynBio granted an exclusive license to us to use any SynBio pre-clinical and clinical data generated by SynBio and to engage in the development and commercialization of drug candidates that may arise from the collaboration in any territory outside of Russia and the CIS based upon the Co-Development Agreement.
We hope and expect to mitigate certain technical and commercial risks of drug development by working in collaboration with SynBio. Under the Co-Development Agreement, SynBio is responsible for progressing six new product candidates through human proof of concept trials in Russia as primary validation for the initiation of EMA/FDA clinical trials by us. The Co-Development Agreement will operate alongside the current arrangements, which we have entered into with Pharmsynthez, an affiliate of SynBio, where a further six product candidates are undergoing clinical development in Russia with the same overall commercial objectives.
The primary goal of the Co-Development Agreement is to research and develop drug candidates for planned commercialization using SynBio and our combined respective expertise and technologies. Drug candidates must meet the success criteria as decided upon by a joint steering committee, which includes representation from both SynBio and us, where we have the right to appoint the Chair who has the casting vote. Once a potential drug candidate is selected, clinical trials will be separately conducted by each company in their respective territories with the goal to achieve regulatory approval of the products for commercial sale.
SynBio is wholly responsible for funding and conducting their own research and clinical development activities in Russia as we are wholly responsible for funding and conducting their own research and clinical development activities in the U.S., Europe and elsewhere ex-Russia and the ex-CIS regions. There are no milestones or other research related payments provided for under the Co-Development Agreement other than fees for the provision of each party’s respective research supplies based on their technology. For the years ended December 31, 2015 and 2014, we have recognized no supply service revenues in connection with the Co-Development Agreement. Among others, the parties may terminate the agreement in relation to a particular product upon 30 days’ written notice, if such party, in its reasonable opinion, believe that a third-party intellectual property right exists, which would have a material effect on the research and/or development of the relevant product. Further, the parties may terminate the agreement if the other party is in material breach of the agreement and, in the case of a breach capable of remedy, the breach is not remedied within 90 days of receiving notice specifying the breach and requiring its remedy, or if the other party becomes insolvent. The parties also may terminate the agreement by immediate written notice to the other party in relation to a specific product such product does not meet the relevant success criteria for the product.
Concurrent with entering into the Co-Development Agreement, we entered into a stock subscription agreement with SynBio pursuant to which we sold SynBio approximately 1.07 million shares of newly issued common stock for cash of approximately $18.6 million making them controlling shareholder of the Company.
In furtherance of our co-development clinical objectives, on December 31, 2014 we granted to SynBio certain warrants that contain vesting triggers based on the achievement by SynBio of certain clinical development objectives within specific timeframes. This grant consisted of a warrant to purchase 204,394 new shares of common stock at an exercise price of $25.41 per share (SynBio 2014 Warrant). Simultaneously with the SynBio 2014 Warrant grant, we granted additional warrants to purchase 9,697 aggregate new shares of common stock to SynBio and Pharmsynthez non-director designees under the same terms and conditions of the SynBio 2014 Warrant. Pharmsynthez is a related party of SynBio and a collaboration partner of ours, as further discussed below. As part of this transaction, the warrant granted to SynBio in 2011 was canceled and of no further force and effect. The SynBio 2014 Warrant expires on December 30, 2019 and no warrants were exercised during the years ended December 31, 2015 and 2014.
On September 23, 2016, SynBio, LLC exchanged 970,000 shares of common stock in the Company for an equal number of shares of Series A Preferred Stock.
SynBio is a related party of ours, with a share ownership of approximately 9.9% of the total issued common stock and all of our outstanding Series A Preferred Stock as of October 20, 2016.
| 84 |
PJSC Pharmsynthez
In November 2011, we entered into a collaborative research and development license agreement with PJSC Pharmsynthez (formerly OJSC Pharmsynthez, the Pharmsynthez Arrangement) pursuant to which we granted an exclusive license to Pharmsynthez to develop, commercialize and market six product candidates based on the Company’s PolyXen and ImuXen technology anywhere within Russia and the CIS. In exchange, Pharmsynthez granted us an exclusive license to use any pre-clinical and clinical data developed by Pharmsynthez, within the scope of the Pharmsynthez Arrangement, and to engage in further research, development and commercialization of drug candidates in any territory outside of Russia and the CIS at the Company’s own expense.
In accordance with the terms of the Pharmsynthez Arrangement, we licensed certain PolyXen and ImuXen technology rights for use in Russia and the CIS as well as certain clinical and research data developed by the Company on the six product candidates to Pharmsynthez.
We hope and expect to mitigate certain risks of drug development by reviewing human clinical data arising out of this collaboration with Pharmsynthez before we take the particular drug candidate into FDA and EMA trials, a strategy designed to mitigate drug development risks. Under the agreement, Pharmsynthez is responsible for progressing six new drug candidates through human proof of concept trials in Russia as primary validation prior to the initiation of EMA/FDA clinical trials by us outside of Russia. The license agreement will operate alongside the current arrangements which we have entered into with SynBio, discussed above. A joint steering committee where we have the right to appoint the Chair who has the casting vote was established to facilitate the communication of scientific data and to assist generally with each party’s research decisions and to monitor research and development progress under the Pharmsynthez Arrangement.
Pharmsynthez is wholly responsible for funding and conducting its own research and clinical development activities in Russia. We are wholly responsible for funding and conducting our own research and clinical development activities in the U.S., Europe and the rest of the world outside of Russia and the ex-CIS regions. There are no milestones or other research related payments provided for under the Pharmsynthez Agreement other than royalties. Among others, the parties may terminate the agreement in relation to a particular product upon 30 days’ written notice, if such party, in its reasonable opinion, believe that a third-party intellectual property right exists, which would have a material effect on the research and/or development of the relevant product. Further, the parties may terminate the agreement if the other party is in material breach of the agreement and, in the case of a breach capable of remedy, the breach is not remedied within 90 days of receiving notice specifying the breach and requiring its remedy, or if the other party becomes insolvent. The parties also may terminate the agreement by immediate written notice to the other party in relation to a specific product such product does not meet the relevant success criteria for the product.
On November 13, 2015, we entered into the Kevelt APA with Kevelt and Pharmsynthez. Pursuant to the Kevelt APA, the Sellers transferred to us certain intellectual property rights held by the Sellers with respect to Virexxa, and we received the worldwide rights to develop, market and license Virexxa for certain uses, except for excluded uses within the CIS, in exchange for, among others, 3,378,788 shares of our common stock. Virexxa is a Phase II oncology drug candidate which is under investigation for the treatment of certain endometrial cancers. We also acquired Kevelt's U.S. Orphan Drug designation for the use of Virexxa in the treatment of progesterone receptor negative endometrial cancer in conjunction with progesterone therapy.
Pharmsynthez is a related party of SynBio, which is related party of the Company. Pharmsynthez has a share ownership of approximately 53.3% of the total issued common stock as of October 20, 2016.
Serum Institute
In the period from 2004 through 2011, we entered into an amended certain license and supply agreements with Serum Institute. The original license agreement with Serum Institute was a collaborative Development and Manufacturing Arrangement (DMA) to develop agreed upon potential commercial product candidates using our PolyXen technology. Serum Institute then endeavored to further develop the potential commercial product candidates and eventually initiate pre-clinical and clinical trials at their own cost. The agreement was amended in 2011, resulting in the surrender of development rights for 14 potential commercial product candidates in 2012, which were vested to Serum Institute under the terms of the previous agreements, back to us.
| 85 |
Following the 2011 amendment, Serum Institute retained an exclusive license to use our PolyXen technology to research and develop one potential commercial product, Polysialylated Erythropoietin (PSA-EPO). Serum Institute will be responsible for conducting all pre-clinical and clinical trials required to achieve regulatory approvals within territories outside of certain predetermined territories assigned to us, which include the U.S., the European Economic Area, and Japan, among other territories, at Serum Institute’s own expense. The royalty payment schedule based on net revenues on the future commercial sales of PSA-EPO under the DMA was also modified as a result of the 2011 amendment. Royalty payments are payable by Serum Institute to us for net sales to certain customers in the Serum Institute sales territory. Royalty payments are payable by us to Serum Institute for net sales received by us over the term of the license. No royalty, revenue or expense was recognized by us related to the Serum Institute arrangement during the years ended December 31, 2015 and 2014. There are no milestone or other research-related payments due under the DMA. Through December 31, 2015, we and Serum Institute continued to engage in research and development activities with no resultant commercial products. Among others, the parties may terminate the license agreement by written notice if the other party is in material breach of the agreement and, in the case of a breach capable of remedy, the breach is not remedied within 90 days of the other party receiving notice specifying the breach and requiring its remedy.
In furtherance of our co-development clinical objectives, on December 31, 2014 we granted to Serum Institute certain warrants that contain vesting triggers based on the achievement by Serum Institute of certain clinical development objectives within specific timeframes. This grant consisted of a warrant to purchase 96,970 new shares of common stock at an exercise price of $25.41 per share (Serum 2014 Warrant). Simultaneously with the Serum 2014 Warrant grant, we granted additional warrants to purchase 4,852 aggregate new shares of common stock to Serum Institute non-director designees under the same terms and conditions of the Serum 2014 Warrant. The Serum 2014 Warrant expires on December 30, 2019 and no warrants were exercised during the years ended December 31, 2015 and 2014.
In addition, the DMA allows for Serum Institute to nominate a non-executive director to our Board of Directors as long as Serum Institute or its subsidiaries holds at least 6% of our common stock. Serum Institute is a related party of ours, with a share ownership of approximately 9.9% of the total issued common stock as of October 20, 2016.
Competition
The pharmaceutical and biotechnology industries are characterized by intense competition and rely heavily on the ability to move quickly, adapt to changing medical and market needs, and to develop and maintain strong intellectual property positions. We believe that the development experience of our scientific and management team, as well as the strength and promise of our product candidates, provide us with a competitive advantage; nevertheless, we face potential competition from a myriad of sources many of which operate with greater resources and more mature products. These include pharmaceutical and biotechnology companies, academic institutions, governmental agencies and public and private research institutions. Competition is intense and expected to increase.
Product and Technology Specific Competition
OncoHist for AML
Our drug candidate OncoHist, if approved, will compete with established therapies for the blood cancer AML. The standard of care is cytarabine in combination with an anthracycline (i.e., daunorucbin).
To our knowledge there is no approved biologic for the treatment of AML. We are aware of certain late-stage development programs that target the same relapse population as our OncoHist program, including BiolineRx, which has multiple clinical trials in process using their peptide BL-8040, and Celator, which is targeting first-line treatment of AML.
Small molecule, FDA-approved drug competition for AML include clofarabine. Other small cytotoxic molecules in development, but not FDA-approved, include azacitidine and decitabine.
In addition, certain immunotherapy and immunomodulating agents in development for other cancers may compete with OncoHist, if approved. These include Kite Pharma and Stemline Therapeutics Inc.
Other novel therapies in development for AML include SL-401 from Stemline Therapeutics Inc., which is a targeted therapy directed to the interleukin-3 receptor delivering truncated diphtheria toxin to the cancer cell.
| 86 |
Virexxa for Endometrial and Ovarian Cancers
Current standard of care treatments for Endometrial Cancer include radiation, surgery as well as certain chemotherapeutic and antineoplastic agents, particularly platinum-based agents, including but not limited to Taxol, carboplatin, doxorubicin, cisplatin, ifosfamide, and topotecan.
A number of additional therapeutic classes are in development worldwide, including antibodies, antibody-drug conjugates (ADCs), and immunotherapies (e.g., bevacizumab and GALE-301/GALE-302, respectively). Additionally, there are a number of targeted agents including inhibitors that target the PI3K/Akt/mTOR pathway (such as AKT inhibitor ARQ-092) and other kinase inhibitors. The aforementioned therapeutics and therapeutic classes may be used either alone or in combination. Companies engaged in clinical development of these products for endometrial cancer include but are not limited to:
| · | Antibodies/Immunotherapies: Galena BioPharma; Merck Sharp & Dohme; Immunogen, Inc. Immunomedics, Inc.; Genentech; Macrogenics; Genmab; Incyte Corporation; and Eisai Inc. Bayer. | |
| · | Targeted Agents: ArQule; AstraZeneca; Novartis; Daiichi Sankyo Inc.; GlaxoSmithKline; and Advenchen Laboratories LLC. |
BAX 826 (PSA rFVIII) for Hemophilia
Shire’s ADYNOVATE, is the current and latest generation long-acting ADVATE. Together, ADVATE and ADYNOVATE make up the bulk of Shire’s the current Hemophilia A portfolio.
Novo Nordisk recently launched a next generation Antihemophilic Factor VIII (Recombinant), Novoeight. It competes with Shire’s pegylated Factor VIII product ADYNOVATE.
Bayer has a next generation drug candidate, BAY94-9027, in Phase III clinical trials. BAY94-9027 has been designed to extend the circulating half-life of rFVIII through site specific attachment of a polyethylene glycol (PEG) polymer to the light chain of the rFVIII molecule, while preserving its full biologic activity.
Biogen Idec’s ELOCTATE Antihemophilic Factor (Recombinant), Fc Fusion Protein, was approved by the FDA in 2014, for the control and prevention of bleeding episodes, perioperative (surgical) management and routine prophylaxis in adults and children with Hemophilia A.
There are other long-acting Factor VIII programs in late-stage development for Hemophilia A patients.
ErepoXen for Anemia
Current commercial products include EPOGEN and Aranesp from Amgen, Procit and Eprex from Johnson & Johnson, and Mircera from Roche Holding Ltd. These established products represent the current standard of care for CKD and ESRD patients.
We understand that many new therapies are erythropoietin stimulating agents, such as HIF-related drug candidates, are designed to be daily oral treatments. Companies currently developing these therapies include Akebia, Bayer HealthCare AG, FibroGen (licensed by Astellas), GlaxoSmithKline plc, and others. We expect certain of these candidates may enter the market as early as 2017.
We believe that other novel therapies are under development that could represent competition with ErepoXen, if approved, include sotatercept from Acceleron Pharma Inc.
| 87 |
PSA for Drug Delivery
Current delivery platforms include PEG, FC-fusion, albumin infusion, HES, depot, CTP-fusion.
Market participants include Nektar’s PEG technology, Flamel’s Medusa platform offering, a hydrogel depot formulation, Versartis’ XTEN technology which recombinant polypeptide fusion protein, nanoparticle technology from Alkermes, Durect Corp’s long-acting technology, Debiopharm Group’s drug delivery based on polylactic-co-glycolic acid (PLGA), and Halozyme’s ENHANZE drug delivery technology platform.
We also expect to compete with academic institutions and other smaller pharmaceutical companies during the drug development stage of our progress. In addition to competing with universities and other research institutions in the development of drug products, therapies, technologies and processes, we may compete with other companies in acquiring rights to products or technologies from universities. There can be no assurance that our products or product candidates will be more effective or achieve greater market acceptance than competitive products, or that these companies will not succeed in developing products and technologies that are more effective than those being developed for us or that would render our products and technologies less competitive or obsolete.
Manufacturing and Supply
We do not own or operate, and currently have no plans to establish, any manufacturing facilities. We currently rely, and expect to continue to rely, on third-parties for the manufacture of our product candidates for clinical testing, as well as for manufacture of any products that we may commercialize. We currently have agreements in place with Serum Institute for production of clinical materials for use in the development of drug candidates involving our PolyXen platform technology. We are currently dependent on SynBio for clinical materials with respect to our OncoHist for AML involving our OncoHist platform technology. This strategy allows us to maintain a more efficient infrastructure, avoid depending on our own manufacturing facility and equipment while simultaneously enabling us to focus our expertise on developing our products. Although we believe we have multiple potential sources for the manufacturing of our product candidates, we currently rely on single manufacturers for the different components our drug candidates. We are investigating second source alternative suppliers for our clinical materials. There can be no assurance that we will be successful or that if a second source is secured that it would be available on commercially reasonable terms or in a timely fashion should any disruption in supply from Serum Institute or SynBio occur.
Sales and Marketing
Given our stage of development, we have not yet established a commercial organization or distribution capabilities, nor have we entered into any partnership or co-promotion arrangements with an established pharmaceutical company. To develop the appropriate commercial infrastructure to launch our product candidates, we may either do so on our own or by establishing alliances with one or more pharmaceutical company collaborators, depending on, among other things, the applicable indications, the related development costs and our available resources.
Government Regulation
General
Government authorities in the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, marketing and export and import of products such as those we are developing. Generally, a new drug must be approved by the FDA through the NDA process and a new biologic must be licensed by the FDA through the BLA process before it may be legally marketed in the United States.
| 88 |
U.S. Regulation
Drug Development Process
In the United States, the FDA regulates drugs under the federal Food, Drug, and Cosmetic Act (FDCA), and in the case of biologics, also under the Public Health Service Act (PHSA), and their implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the FDA's refusal to approve pending applications, withdrawal of an approval, license revocation, a clinical hold, warning letters or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us.
The process required by the FDA before a drug or biologic may be marketed in the United States generally involves the following:
| · | completion of preclinical laboratory tests, animal studies and formulation studies in accordance with GLP regulations and other applicable regulations; | |
| · | submission to the FDA of an IND, which must become effective before human clinical trials may begin; | |
| · | performance of adequate and well-controlled human clinical trials in accordance with Good Clinical Practice (GCP) regulations to establish the safety and efficacy of the proposed drug for its intended use; | |
| · | submission to the FDA of an NDA or BLA; | |
| · | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with current Good Manufacturing Practices (cGMP) requirements to assure that the facilities, methods and controls are adequate to preserve the drug's identity, strength, quality and purity; and | |
| · | FDA review and approval of the NDA or BLA. |
Once a pharmaceutical candidate is identified for development, it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. The sponsor will also include a protocol detailing, among other things, the objectives of the first phase of the clinical trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated, if the first phase lends itself to an efficacy evaluation. Some preclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Clinical holds also may be imposed by the FDA at any time before or during clinical trials due to safety concerns about ongoing or proposed clinical trials or noncompliance with specific FDA requirements, and the trials may not begin or continue until the FDA notifies the sponsor that the hold has been lifted.
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with GCP regulations. They must be conducted under protocols detailing the objectives of the trial, dosing procedures, subject selection and exclusion criteria and the safety and effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND, and timely safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events. An institutional review board (IRB) at each institution participating in the clinical trial must review and approve each protocol before a clinical trial commences at that institution and must also approve the information regarding the trial and the consent form that must be provided to each trial subject or his or her legal representative, monitor the study until completed and otherwise comply with IRB regulations.
| 89 |
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| · | Phase I: The product candidate is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, such as cancer, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients. |
| · | Phase II: This phase involves clinical trials in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and appropriate dosage. |
| · | Phase III: Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These clinical trials are intended to establish the overall risk-benefit ratio of the product candidate and provide, if appropriate, an adequate basis for product labeling. |
Post-approval trials, sometimes referred to as Phase 4 studies, may be conducted after initial marketing approval. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, the FDA may mandate the performance of Phase IV clinical trials as a condition of approval of an NDA or BLA.
The FDA or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB's requirements or if the drug has been associated with unexpected serious harm to patients. In addition, some clinical trials are overseen by an independent group of qualified experts organized by the sponsor, known as a data safety monitoring board or committee. Depending on its charter, this group may determine whether a trial may move forward at designated check points based on access to certain data from the trial.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug. In addition, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
While the IND is active and before approval, progress reports summarizing the results of the clinical trials and nonclinical studies performed since the last progress report must be submitted at least annually to the FDA, and written IND safety reports must be submitted to the FDA and investigators for serious and unexpected suspected adverse events, findings from other studies suggesting a significant risk to humans exposed to the same or similar drugs, findings from animal or in vitro testing suggesting a significant risk to humans, and any clinically important increased incidence of a serious suspected adverse reaction compared to that listed in the protocol or investigator brochure.
There are also requirements governing the reporting of ongoing clinical trials and completed trial results to public registries. Sponsors of certain clinical trials of FDA-regulated products are required to register and disclose specified clinical trial information, which is publicly available at www.clinicaltrials.gov. Information related to the product, patient population, phase of investigation, trial sites and investigators and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to discuss the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed until the new product or new indication being studied has been approved.
| 90 |
United States Market Approval Process
The results of product development, preclinical and other non-clinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling and other relevant information are submitted to the FDA as part of an NDA or BLA requesting approval to market the product. The submission of an NDA or BLA is subject to the payment of user fees; a waiver of such fees may be obtained under certain limited circumstances. The FDA reviews all NDAs and BLAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. The FDA may request additional information rather than accept an NDA or BLA for filing. In this event, the NDA or BLA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing.
Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA may refer the NDA or BLA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. The approval process is lengthy and often difficult, and the FDA may refuse to approve an NDA or BLA if the applicable regulatory criteria are not satisfied or may require additional clinical or other data and information. Even if such data and information are submitted, the FDA may ultimately decide that the NDA or BLA does not satisfy the criteria for approval. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product's identity, strength, quality and purity. The FDA reviews a BLA to determine, among other things whether the product is safe, pure and potent and the facility in which it is manufactured, processed, packed or held meets standards designed to assure the product's continued safety, purity and potency. Before approving an NDA or BLA, the FDA will inspect the facility or facilities where the product is manufactured.
After the FDA evaluates an NDA or BLA, it will issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the drug with prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete and the application will not be approved in its present form. A Complete Response Letter usually describes the specific deficiencies in the NDA or BLA identified by the FDA and may require additional clinical data, such as an additional pivotal Phase 3 trial or other significant and time-consuming requirements related to clinical trials, nonclinical studies or manufacturing. If a Complete Response Letter is issued, the sponsor must resubmit the NDA or BLA, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information are submitted, the FDA may decide that the NDA or BLA does not satisfy the criteria for approval.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. In addition, the FDA may require a sponsor to conduct Phase 4 testing, which involves clinical trials designed to further assess a drug's safety and effectiveness after NDA or BLA approval, and may require testing and surveillance programs to monitor the safety of approved products which have been commercialized. The FDA may also place other conditions on approval including the requirement for a risk evaluation and mitigation strategy (REMS) to assure the safe use of the drug. If the FDA concludes a REMS is needed, the sponsor of the NDA or BLA must submit a proposed REMS. The FDA will not approve the NDA or BLA without an approved REMS, if required. A REMS could include medication guides, physician communication plans or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of products. Marketing approval may be withdrawn for noncompliance with regulatory requirements or if problems occur following initial marketing.
| 91 |
Orphan Drug Act
The Orphan Drug Act provides incentives to manufacturers to develop and market drugs or biologics for rare diseases and conditions affecting fewer than 200,000 persons in the U.S. at the time of application for Orphan Drug Designation, or for a patient population greater than 200,000 in the United States where there is no reasonable expectation that the cost of developing the drug or biologic will be recovered from sales in the United States. The first developer to receive FDA marketing approval for an orphan drug is entitled to a seven year exclusive marketing period in the U.S. for that product. However, a drug that the FDA considers to be clinically superior to, or different from, another approved orphan drug, even though for the same indication, may also obtain approval in the U.S. during the seven year exclusive marketing period. In addition, holders of exclusivity for orphan drugs are expected to assure the availability of sufficient quantities of their orphan drugs to meet the needs of patients. Failure to do so could result in the withdrawal of marketing exclusivity for the drug.
Pediatric Information
Under the Pediatric Research Equity Act of 2007 (PREA), NDAs or BLAs or supplements to NDAs or BLAs must contain data to assess the safety and effectiveness of the drug for the claimed indication(s) in all relevant pediatric sub-populations and to support dosing and administration for each pediatric sub-population for which the drug is safe and effective. The FDA may grant deferrals for submission of data or full or partial waivers. Unless otherwise required by regulation, PREA does not apply to any drug for an indication for which orphan drug designation has been granted. The Best Pharmaceuticals for Children Act (BPCA), provides sponsors of NDAs with an additional six month period of market exclusivity for all unexpired patent or non-patent exclusivity on all forms of the drug containing the active moiety if the sponsor submits results of pediatric studies specifically requested by the FDA under BPCA within required timeframes. The Biologics Price Competition and Innovation Act provides sponsors of BLAs an additional six month extension for all unexpired non-patent market exclusivity on all forms of the biologic containing the active moiety pursuant to the BPCA if the conditions under the BPCA are met.
The Food and Drug Administration Safety and Innovation Act (FDASIA), which was signed into law on July 9, 2012, amended the FDCA. FDASIA requires that a sponsor who is planning to submit a marketing application for a drug or biological product that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration submit an initial Pediatric Study Plan (PSP), within sixty days of an end-of-Phase II meeting or as may be agreed between the sponsor and FDA. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. FDA and the sponsor must reach agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from nonclinical studies, early phase clinical trials, and/or other clinical development programs.
Expedited Development and Review Programs
The FDA has a Fast Track program that is intended to expedite or facilitate the process for reviewing new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast Track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a new drug or biologic may request the FDA to designate the drug or biologic as a Fast Track product at any time during the clinical development of the product. Unique to a Fast Track product, the FDA may consider for review sections of the marketing application on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the application, the FDA agrees to accept sections of the application and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the application.
| 92 |
Any product submitted to the FDA for marketing, including under a Fast Track program, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Fast Track designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process. Any product is eligible for priority review if it has the potential to provide safe and effective therapy where no satisfactory alternative therapy exists or a significant improvement in the treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new drug or biological product designated for priority review in an effort to facilitate the review. Additionally, a product may be eligible for accelerated approval. Drug or biological products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, which means that they may be approved on the basis of adequate and well-controlled clinical trials establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, the FDA may require that a sponsor of a drug or biological product receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product. If the FDA concludes that a drug shown to be effective can be safely used only if distribution or use is restricted, it will require such post-marketing restrictions as it deems necessary to assure safe use of the drug, such as distribution restricted to certain facilities or physicians with special training or experience; or distribution conditioned on the performance of specified medical procedures.
FDASIA established a new category of drugs and biologics referred to as "breakthrough therapies" that may be eligible to receive Breakthrough Therapy Designation. A sponsor may seek FDA designation of a drug or biologic candidate as a "breakthrough therapy" if the product is intended, alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The designation includes all of the Fast Track program features, as well as more intensive FDA interaction and guidance. The Breakthrough Therapy Designation is a distinct status from both accelerated approval and priority review, which can also be granted to the same drug if relevant criteria are met. If a product is designated as breakthrough therapy, the FDA will expedite the development and review of such drug. All requests for breakthrough therapy designation will be reviewed within 60 days of receipt, and the FDA will either grant or deny the request.
Post-Approval Requirements
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements or standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. After approval, some types of changes to the approved product, such as adding new indications, certain manufacturing changes and additional labeling claims, are subject to further FDA review and approval. Drug and biologics manufacturers and other entities involved in the manufacture and distribution of approved drugs and biologics are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP regulations and other laws and regulations.
US Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of the FDA approval of our drug candidates, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA or BLA plus the time between the submission date of an NDA or BLA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we intend to apply for restoration of patent term for one of our currently owned or licensed patents to add patent life beyond its current expiration date, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant NDA or BLA.
| 93 |
Marketing exclusivity provisions under the FDCA can also delay the submission or the approval of certain marketing applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to obtain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application (ANDA), or a 505(b)(2) NDA submitted by another company for another drug based on the same active moiety, regardless of whether the drug is intended for the same indication as the original innovator drug or for another indication, where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement to one of the patents listed with the FDA by the innovator NDA holder. The FDCA also provides three years of marketing exclusivity for an NDA, or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example new indications, dosages or strengths of an existing drug. This three-year exclusivity covers only the modification for which the drug received approval on the basis of the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the active agent for the original indication or condition of use. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the pre-clinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Pediatric exclusivity is another type of regulatory market exclusivity in the United States under the BPCA. Pediatric exclusivity provides for an additional six months of marketing exclusivity if a sponsor conducts clinical trials in children as addressed in the section named “Pediatric Information” above. In addition, orphan drug exclusivity, as described above, may offer a seven-year period of marketing exclusivity, except in certain circumstances.
Biosimilars and Exclusivity
The Affordable Care Act includes a subtitle called the Biologics Price Competition and Innovation Act of 2009 (BPCIA), which created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. To date, only two biosimilars have been licensed under the BPCIA, although numerous biosimilars have been approved in Europe. The FDA has issued several guidance documents outlining an approach to review and approval of biosimilars.
Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate that it can be expected to produce the same clinical results as the reference product in any given patient and, for products that are administered multiple times to an individual, the biologic and the reference biologic may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. However, complexities associated with the larger, and often more complex, structures of biological products, as well as the processes by which such products are manufactured, pose significant hurdles to implementation of the abbreviated approval pathway that are still being addressed by the FDA.
Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until twelve years from the date on which the reference product was first licensed. During this twelve-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing the sponsor's own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. The BPCIA also created certain exclusivity periods for biosimilars approved as interchangeable products. At this juncture, it is unclear whether products deemed "interchangeable" by the FDA will, in fact, be readily substituted by pharmacies, which are governed by state pharmacy law.
The BPCIA is complex and only beginning to be interpreted and implemented by the FDA. In addition, recent government proposals have sought to reduce the twelve-year reference product exclusivity period. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. As a result, the ultimate impact, implementation and meaning of the BPCIA is subject to significant uncertainty.
| 94 |
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our product candidates.
Whether or not we obtain FDA approval for our product candidates, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product candidates in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In the European Union, for example, a clinical trial authorization (CTA) must be submitted to each country's national health authority and an independent ethics committee, much like the FDA and the IRB, respectively. Once the CTA is approved in accordance with a country's requirements, clinical study development may proceed.
The requirements and process governing the conduct of clinical trials, product approval and licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain regulatory approval of an investigational drug or biological product under European Union regulatory systems, we must submit a marketing authorization application. The application used to file the NDA or BLA in the United States is similar to that required in the European Union, with the exception of, among other things, country-specific document requirements. The European Union also provides opportunities for market exclusivity. For example, in the European Union, upon receiving marketing authorization, new chemical entities generally receive eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents regulatory authorities in the European Union from referencing the innovator's data to assess a generic application. During the additional two-year period of market exclusivity, a generic marketing authorization can be submitted, and the innovator's data may be referenced, but no generic product can be marketed until the expiration of the market exclusivity. However, there is no guarantee that a product will be considered by the European Union's regulatory authorities to be a new chemical entity, and products may not qualify for data exclusivity. Products receiving orphan designation in the European Union can receive ten years of market exclusivity, during which time no similar medicinal product for the same indication may be placed on the market. An orphan product can also obtain an additional two years of market exclusivity in the European Union for pediatric studies. No extension to any supplementary protection certificate can be granted on the basis of pediatric studies for orphan indications.
The criteria for designating an "orphan medicinal product" in the European Union are similar in principle to those in the United States. Under Article 3 of Regulation (EC) 141/2000, a medicinal product may be designated as orphan if (1) it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition; (2) either (a) such condition affects no more than five in 10,000 persons in the European Union when the application is made, or (b) the product, without the benefits derived from orphan status, would not generate sufficient return in the European Union to justify investment; and (3) there exists no satisfactory method of diagnosis, prevention or treatment of such condition authorized for marketing in the European Union, or if such a method exists, the product will be of significant benefit to those affected by the condition, as defined in Regulation (EC) 847/2000. Orphan medicinal products are eligible for financial incentives such as reduction of fees or fee waivers and are, upon grant of a marketing authorization, entitled to ten years of market exclusivity for the approved therapeutic indication. The application for orphan drug designation must be submitted before the application for marketing authorization. The applicant will receive a fee reduction for the marketing authorization application if the orphan drug designation has been granted, but not if the designation is still pending at the time the marketing authorization is submitted. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
| 95 |
The 10-year market exclusivity may be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan designation, for example, if the product is sufficiently profitable not to justify maintenance of market exclusivity. In addition, marketing authorization may be granted to a similar product for the same indication at any time if:
| · | the second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior; | |
| · | the applicant consents to a second orphan medicinal product application; or | |
| · | the applicant cannot supply enough orphan medicinal product. |
For other countries outside of the European Union, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical studies, product licensing or approval, pricing and reimbursement vary from country to country. In all cases, again, the clinical studies are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Other Regulatory Matters
Manufacturing, sales, promotion and other activities following product approval are also subject to regulation by numerous regulatory authorities in addition to the FDA, including, in the United States, the Centers for Medicare & Medicaid Services, other divisions of the Department of Health and Human Services, the Drug Enforcement Administration, the Consumer Product Safety Commission, the Federal Trade Commission, the Occupational Safety & Health Administration, the Environmental Protection Agency and state and local governments. In the United States, sales, marketing and scientific/educational programs must also comply with state and federal fraud and abuse laws, including state and federal anti-kickback, false claims, data privacy and security and physician payment transparency laws. Pricing and rebate programs must comply with the Medicaid rebate requirements of the U.S. Omnibus Budget Reconciliation Act of 1990 and more recent requirements in the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, collectively the Affordable Care Act. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. The handling of any controlled substances must comply with the U.S. Controlled Substances Act and Controlled Substances Import and Export Act. Products must meet applicable child-resistant packaging requirements under the U.S. Poison Prevention Packaging Act. Manufacturing, sales, promotion and other activities are also potentially subject to federal and state consumer protection and unfair competition laws.
The distribution of pharmaceutical products is subject to additional requirements and regulations, including extensive record-keeping, licensing, storage and security requirements intended to prevent the unauthorized sale of pharmaceutical products.
The failure to comply with regulatory requirements may subject us to possible legal or regulatory action. Depending on the circumstances, failure to meet applicable regulatory requirements can result in criminal prosecution, fines or other penalties, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of product approvals, or refusal to allow a firm to enter into supply contracts, including government contracts. In addition, even if a firm complies with FDA and other requirements, new information regarding the safety or efficacy of a product could lead the FDA to modify or withdraw product approval. Prohibitions or restrictions on sales or withdrawal of future products marketed by us could materially affect our business in an adverse way.
Changes in regulations, statutes or the interpretation of existing regulations could impact our business in the future by requiring, for example: (i) changes to our manufacturing arrangements; (ii) additions or modifications to product labeling; (iii) the recall or discontinuation of our products; or (iv) additional record-keeping requirements. If any such changes were to be imposed, they could adversely affect the operation of our business.
| 96 |
Environmental Regulation
In addition to being subject to extensive regulation by the FDA, we must also comply with environmental regulation insofar as such regulation applies to us or our drug candidates. Our costs of compliance with environmental regulation as applied to similar pharmaceutical companies are minimal, since we do not currently, nor do we intend to, engage in the manufacturing of any of our drug candidates. We currently use unaffiliated manufacturers to produce all of our drug candidate material and receive final material from such manufacturer, without any involvement on our part in the manufacturing process at any stage of the process.
Although we believe that our safety procedures for using, handling, storing and disposing of our product candidate materials comply with the environmental standards required by state and federal laws and regulations, we cannot completely eliminate the risk of accidental contamination or injury from these materials. We do not carry a specific insurance policy to mitigate this risk to us or to the environment.
Coverage and Reimbursement
Sales of our products will depend, in part, on the extent to which our products will be covered by third-party payors, such as government health programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly reducing reimbursements for medical products and services. Additionally, the containment of healthcare costs has become a priority of federal and state governments, and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Decreases in third-party reimbursement for our product candidates or a decision by a third-party payor to not cover our product candidates could reduce physician usage of the product candidates and have a material adverse effect on our sales, results of operations and financial condition.
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA), established the Medicare Part D program to provide a voluntary prescription drug benefit to Medicare beneficiaries. Under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities which provide coverage of outpatient prescription drugs. Unlike Medicare Part A and B, Part D coverage is not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. However, Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, though not necessarily all the drugs in each category or class. Any formulary used by a Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee. Government payment for some of the costs of prescription drugs may increase demand for products for which we receive marketing approval. However, any negotiated prices for our products covered by a Part D prescription drug plan will likely be lower than the prices we might otherwise obtain. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own payment rates. Any reduction in payment that results from the MMA may result in a similar reduction in payments from non-governmental payors.
The American Recovery and Reinvestment Act of 2009 provides funding for the federal government to compare the effectiveness of different treatments for the same illness. The plan for the research was published in 2012 by the Department of Health and Human Services, the Agency for Healthcare Research and Quality and the National Institutes for Health, and periodic reports on the status of the research and related expenditures will be made to Congress. Although the results of the comparative effectiveness studies are not intended to mandate coverage policies for public or private payors, it is not clear what effect, if any, the research will have on the sales of our product candidates, if any such products or the condition that they are intended to treat is the subject of a trial. It is also possible that comparative effectiveness research demonstrating benefits in a competitor’s product could adversely affect the sales of our product candidates. If third-party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
| 97 |
The Affordable Care Act, or ACA, enacted in March 2010, is expected to have a significant impact on the health care industry. The ACA expands coverage for the uninsured while at the same time is expected to contain overall healthcare costs. With regard to pharmaceutical products, among other things, the ACA expands and increases industry rebates for drugs covered under Medicaid programs and makes changes to the coverage requirements under the Medicare Part D program. Under the ACA, pharmaceutical manufacturers are required to track certain financial arrangements with physicians and teaching hospitals, including any “transfer of value” made or distributed to such entities, as well as any investment interests held by physicians and their immediate family members. Manufacturers were required by ACA to begin tracking this information in 2013 and began reporting this information to CMS in 2014. We cannot predict the full impact of the ACA on pharmaceutical companies.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. On August 2, 2011, the Budget Control Act of 2011 among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, started in April 2013. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012 (ATRA), which delayed for another two months the budget cuts mandated by these sequestration provisions of the Budget Control Act of 2011. The ATRA, among other things, also reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. We expect that additional federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, and in turn could significantly reduce the projected value of certain development projects and reduce our profitability.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the United States and generally tend to be significantly lower.
Reliance on Principal Customer
Since August 2005, Shire has been our principal customer, accounting for the substantial portion of our revenue, through up-front payments and fee for services. See “Business - Significant Co-Development Collaborations and Strategic Arrangements” on page 83 for further information regarding the importance of our relationship with Shire.
Employees
As of June 30, 2016, we employed six (6) persons full time and three (3) persons part-time. We are not a party to any collective bargaining agreement with our employees; nor are any of our employees a member of any labor unions. We are subject to certain statutory and contractual obligations in instances where we terminate U.K. based employees. These obligations, which are ordinary and customary in the U.K., generally range from one to six months wages for terminated employees and would not be expected to represent a material adverse effect to us.
To complement our own expert professional staff, we utilize specialists in regulatory affairs, pharmacovigilance, process engineering, manufacturing, quality assurance, clinical development and business development. These individuals include scientific advisors as well as independent consultants.
| 98 |
Facilities
We lease a facility consisting of approximately 4,000 square feet in the Ledgemont Research Center in Lexington, Massachusetts. The premises are divided into approximately 50% laboratory and 50% office space. The lease provides for an initial term of 61 months commencing January 2014, with an extension option of one additional five year term. We believe that this space is adequate for our current needs, and that if additional space is required, it can be obtained at commercially reasonable terms either within the Ledgemont Research Center or nearby.
Legal Proceedings
We are not currently party to any material legal proceedings.
| 99 |
Management
Directors and Executive Officers
The following table sets forth information regarding our executive officers and directors as of October 20, 2016:
| Name | Age | Position |
| Executive Officers and Employee Directors | ||
| M. Scott Maguire | 53 | President, Chief Executive Officer and Director |
| NonEmployee Directors | ||
| Firdaus Jal Dastoor, FCS(1)(2) | 64 | Director |
| Dr. Roger Kornberg | 69 | Director |
| Roman Knyazev(3) | 36 | Director |
| Darlene Deptula-Hicks(1)(2)(3) | 59 | Director |
| Jeffrey F. Eisenberg | 50 | Director |
(1) Member of the Audit Committee.
(2) Member of the Compensation Committee.
(3) Member of the Nominating and Corporate Governance Committee.
Executive Officers and Employee Directors
M. Scott Maguire has served as our President, Chief Executive Officer and a member of our Board since January 2014, having been appointed pursuant to terms included in the Company’s acquisition of Xenetic U.K. Mr. Maguire served as the Chief Executive Officer of Xenetic U.K. from April 2004 to January 2014. He has a background in life science and healthcare investment banking advising U.S. and European companies on capital raisings and commercial development. Mr. Maguire began his banking career with Merrill Lynch in 1987 in New York. After receiving his M.B.A. from the Babson Graduate School in 1993, he joined the healthcare division of W.R. Grace National Medical Care (NMC) where he helped develop the international healthcare division. During his time in charge of international business development, he helped double NMC’s international revenues through mergers and acquisitions. In 1996 he co-founded the Arthur Andersen global healthcare corporate finance practice based in London. He subsequently built the practice to include a staff of 36 across the U.S. and Europe, and was elevated to the role of managing director. Mr. Maguire is currently a director of Healthcare Capital Partners Limited, a healthcare corporate finance and proprietary investment boutique he co-founded in 2002 and a non-executive director of Renal Services (UK) Limited, a company focused on dialysis service provision in the U.K. We believe Mr. Maguire’s experience within the biotechnology sector and as an executive and member of other boards of directors provides him with the appropriate set of skills to serve as a member of our Board.
Directors
Firdaus Jal Dastoor, FCS, has served as a member of our Board since January 2014 pursuant to terms included in the Company’s acquisition of Xenetic U.K. Mr. Dastoor was appointed non-executive Director of Xenetic U.K. in July 2007. He is a Fellow Member of The Institute of Company Secretaries of India since 2008. He was Company Secretary of the Poonawalla Group until 1994. He then took on assignments involved in business development strategies and operations, including Serum Institute, Eureka Finvest Pvt, Ltd, EagleBurgmann India Pvt, Ltd. And PPCE Pvt, Ltd. Mr. Dastoor is on the board of several companies operating in the field of engineering products, life sciences and biotech, international trade, financial services and quality standards certifications. Currently, he is a Group Director of the Poonawalla Group of Companies in charge of Finance and Corporate Affairs. We believe Mr. Dastoor’s experience in the field of life sciences and biotechnology, finance and business development provides him with the appropriate set of skills to serve as a member of our Board.
| 100 |
Dr. Roger Kornberg has served as a member of our Board since February 2016. Dr. Kornberg is a member of the U.S. National Academy of Sciences and the Winzer Professor of Medicine in the Department of Structural Biology at Stanford University. He earned his bachelor's degree in chemistry from Harvard University in 1967 and his Ph.D. in chemical physics from Stanford in 1972. He became a postdoctoral fellow at the Laboratory of Molecular Biology in Cambridge, England and then an assistant professor of biological chemistry at Harvard Medical School in 1976, before moving to his present position as professor of structural biology at Stanford Medical School in 1978. In 2006, Dr. Kornberg was awarded the Nobel Prize in Chemistry in recognition for his studies of the molecular basis of Eukaryotic Transcription, the process by which DNA is copied to RNA. Dr. Kornberg is also the recipient of several awards, including the 2001 Welch Prize, the highest award granted in the field of chemistry in the United States, and the 2002 Leopald Mayer Prize, the highest award granted in the field of biomedical sciences from the French.
Roman Knyazev has served as a member of our Board since April 2014. Mr. Knyazev has served in various positions, most recently as Senior Investment Manager, of Rusnano Moscow since 2009. Mr. Knyazev serves on the board of Nanolek, PETAR, Pharmsynthez, Lipoxen Technologies, Ltd and SynBio. From 2003 to 2008, Mr. Knyazev served as Chief Financial Officer of Biotec Pharma Moscow. From 2007 to 2009 he was a manager at PricewaterHouseCoopers. Mr. Knyazev is a Fellow of the Kauffman Fellows Program, and he is a certified management accountant. We believe Mr. Knyazev’s experience in clinical stage biotechnology companies provides him with the appropriate set of skills to serve as a member of our Board.
Darlene Deptula-Hicks has served as a member of our Board since April 2014. Since September 2015, Ms. Deptula-Hicks has served as the Chief Financial Officer and Senior Vice President of Pieris Pharmaceuticals, Inc. (NASDAQ: PIRS). In November 2014, Ms. Deptula-Hicks was engaged as a financial consultant at Pieris Pharmaceuticals, Inc. pursuant to a consulting agreement with the financial advisory firm of Danforth Advisors, LLC. From June 2012, Ms. Deptula-Hicks served as Vice President and Chief Financial Officer of Microline Surgical, Inc. From 2006 to 2011, Ms. Deptula-Hicks was the Vice President, Chief Financial Officer, Treasurer and Secretary of ICAD, Inc. Ms. Deptula-Hicks also serves on the board of directors of USfalcon. She received her Bachelor of Science in Accounting from Southern New Hampshire University and her M.B.A. from Rivier College. We believe Ms. Deptula-Hicks’ extensive financial experience in fund raising, mergers, public companies and life sciences provides her with the appropriate set of skills to serve as a member of our Board.
Jeffrey F. Eisenberg has served as a member of our Board since July 2016. From 1998 to 2016, Mr. Eisenberg worked at Noven Pharmaceuticals, Inc., a Miami, Florida based subsidiary of Hisamitsu Pharmaceutical, Inc. Most recently, from 2009-2016, Mr. Eisenberg worked as Noven’s President, CEO and as a member of its board of directors. Currently, Mr. Eisenberg is a member of the board of directors of Mabvax Therapeutics, Inc., a San Diego, California based publicly traded clinical-stage Biopharmaceutical Company focused on discovering and developing innovative vaccine and monoclonal antibody-based therapeutics for the diagnosis and treatment of cancer.Mr. Eisenberg obtained his Juris Doctor at Columbia University Law School, New York, NY, and a Bachelor of Science in Economics from the Wharton School, University of Pennsylvania, Philadelphia, PA.. We believe Mr. Eisenberg’s extensive life science executive experience and leadership experience in the areas of R&D, operations, manufacturing/quality, business development, strategic partnering, product development, commercialization, and talent management provides him with the appropriate set of skills to serve as a member of our Board.
The Board of Directors
The Board of Directors held six (6) meetings during the fiscal year ended December 31, 2015. During the 2015 fiscal year, all Board members attended at least seventy-five percent (75%) of the total number of Board meetings held during the period he or she was a director. Currently, the Board consists of six members: M. Scott Maguire, Firdaus Jal Dastoor, Roman Knyazev, Dr. Roger Kornberg, Darlene Deptula-Hicks, and Jeffrey F. Eisenberg. The Chairman of the Board position remains vacant as of the date of this filing. Members shall hold office until their successors have been duly elected and qualified. Vacancies on the Board of Directors resulting from death, resignation, disqualification, removal, or other causes can be filled by the affirmative vote of a majority of the directors then in office. Any director so elected, shall hold office for the remainder of the full term of the director for which the vacancy was created or occurred and until such director’s successor shall have been duly elected and qualified.
| 101 |
Board of Directors’ Role in Risk Oversight
Our Board is responsible for consideration and oversight of risks to us, and is responsible for ensuring that material risks are identified and managed appropriately, including the evaluation of our risk assessment and risk management policies. In fulfilling this role, the Board receives reports directly from us. In addition, the Board reviews areas of our material risk, including operational, financial, legal, regulatory and strategic risks. The Board also considers the risks associated with our compensation policies and practices, oversees risks associated with our governance structure and processes and annually reviews our organizational documents and other policies. The Board also considers specific risk topics in connection with strategic planning and other matters.
Director Independence
In connection with this offering, we intend to list our common stock on The NASDAQ Capital Market. Under the rules of The NASDAQ Capital Market, independent directors must comprise a majority of a listed company’s board of directors. In addition, the rules of The NASDAQ Capital Market require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and corporate governance committees be independent. Under the rules of The NASDAQ Capital Market, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
Our board of directors has undertaken a review of the independence of each director and considered whether each director has a material relationship with us that could compromise his ability to exercise independent judgment in carrying out his responsibilities. As a result of this review, our board of directors determined that each of Mr. Dastoor, FCS, Dr. Kornberg, Ms. Deptula-Hicks and Mr. Eisenberg are independent directors as defined under the applicable rules and regulations of the Securities and Exchange Commission (SEC), and the listing requirements and rules of The NASDAQ Capital Market. In making these determinations, our board of directors reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and relationships as they may relate to us and our management, including the beneficial ownership of our capital stock by each nonemployee director and the transactions involving them described in the section titled “Certain Relationships and Related Party Transactions.”
Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended (Exchange Act). In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors or any other board committee: (1) accept, directly or indirectly, any consulting, advisory or other compensatory fee from the listed company or any of its subsidiaries; or (2) be an affiliated person of the listed company or any of its subsidiaries. We intend to satisfy the audit committee independence requirements of Rule 10A-3 upon the completion of this offering.
Committees of the Board
Our Board has designated the following three standing committees: the Audit Committee, the Compensation Committee and the Nominating and Corporate Governance Committee. It has adopted charters to govern the conduct, authority and responsibilities of each of these committees. Our board of directors may establish other committees to facilitate the management of our business. The composition and functions of each committee are described below.
| 102 |
Audit Committee
The Audit Committee is responsible for making recommendations to the Board concerning the selection and engagement of independent accountants and for reviewing the scope of the annual audit, audit fees, results of the audit and auditor independence. The Audit Committee also reviews and discusses with management and the Board such matters as accounting policies, internal accounting controls and procedures for preparation of financial statements. The Audit Committee (formed in August 2014) held four (4) meetings during 2015. Prior to the formation of the Audit Committee, the Board of Directors as a whole performed the equivalent function. The Company’s Audit Committee Charter provides that the Audit Committee shall consist of at least three members. The resignation of former Director and Audit Committee member, Mark Leuchtenberger, on April 16, 2015 has created a vacancy on the Audit Committee that has not been filled as of the date of this filing. Any vacancy occurring on the Audit Committee may be filled only by the Board. A copy of the current Audit Committee Charter is available on the Company’s website, WWW.XENETICBIO.COM. The Audit Committee Charter satisfies the applicable standards of the SEC and NASDAQ. The adequacy of the Audit Committee Charter shall be reassessed annually.
The current members of our Audit Committee are Ms. Deptula-Hicks and Mr. Dastoor, FCS. Ms. Deptula-Hicks serves as the chairperson of the committee. All members of our Audit Committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and NASDAQ. In August 2014, the Board of Directors determined that Ms. Deptula-Hicks is an “audit committee financial expert” within the meaning of applicable regulations adopted by the SEC and has the requisite financial sophistication as defined under the applicable rules and regulations of NASDAQ. Under the rules of the SEC, members of the Audit Committee must also meet heightened independence standards. However, so long as at least one member of the Audit Committee satisfies the heightened audit committee independence standards on the date of the effectiveness of the registration statement of which this prospectus forms a part, a majority of members of the audit committee may be exempt from the heightened audit committee independence standards for 90 days from such date and a minority of members of the audit committee may be exempt from the heightened audit committee independence standards for one year from such date. Our Board has determined that each of the members of our Audit Committee is independent under the applicable rules of NASDAQ.
Compensation Committee
Our Compensation Committee reviews and recommends policies relating to compensation and benefits of our officers and employees. The Compensation Committee held two (2) meetings during 2015. The Compensation Committee reviews and sets or makes recommendations to our Board regarding the compensation of our Chief Executive Officer and other executive officers. The compensation committee also reviews and makes recommendations to our Board regarding director compensation. In addition, the compensation committee reviews and approves or makes recommendations to our Board regarding our incentive compensation and equity-based plans. The Compensation Committee periodically reviews and evaluates the performance of the Compensation Committee and its members and must annually review and reassess the Compensation Committee charter and recommend any changes to our Board. The current members of our Compensation Committee are Ms. Deptula-Hicks and Mr. Dastoor, FCS.
Ms. Deptula-Hicks serves as the chairperson of the committee. Each of the members of our Compensation Committee is independent under the applicable rules and regulations of NASDAQ and is an "outside director" as that term is defined in Section 162(m) of the Internal Revenue Code of 1986, as amended (162(m)). Each of Ms. Deptula-Hicks and Mr. Dastoor, FCS is also a “nonemployee director” as defined in Rule 16b¬3 under the Exchange Act. A copy of the current Compensation Committee Charter is available on the Company’s website, WWW.XENETICBIO.COM. The adequacy of the Compensation Committee Charter shall be reassessed annually.
| 103 |
Nominating and Corporate Governance Committee
Our Nominating and Corporate Governance Committee is responsible for identifying individuals qualified to become board members, consistent with criteria approved by the Board, and recommending that the Board select the director nominees for election at each annual meeting of stockholders. In addition, this committee is also responsible for developing and recommending to the Board a set of corporate governance guidelines, periodically reviewing such guidelines and recommending any changes thereto, and overseeing the evaluation of the Board and management. The current members are Ms. Deptula-Hicks and Mr. Knyazev. Each of the members of our nominating and corporate governance committee is an independent director under the applicable rules and regulations of NASDAQ relating to nominating and corporate governance committee independence. The Nomination and Corporate Governance Committee (formed in August 2014) held one (1) meeting during 2015. Prior to the formation of this committee, the Board of Directors as a whole performed the equivalent function. The Nomination and Corporate Governance Committee Charter provides that the committee shall consist of no fewer than two members. A copy of the current Nominating and Corporate Governance Committee Charter is available on the Company’s website, WWW.XENETICBIO.COM. The adequacy of the Compensation Committee Charter shall be reassessed annually.
Compensation Committee Interlocks and Insider Participation
None of the members of our Compensation Committee has at any time been one of our officers or employees. None of our executive officers currently serves, or in the past fiscal year has served, as a member of the Board or Compensation Committee of any entity that has one or more executive officers on our board of directors or Compensation Committee.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. Following the consummation of this offering, the code of business conduct and ethics will be available on our website. We expect that any amendments to the code, or any waivers of its requirements, will be disclosed on our website, WWW.XENETICBIO.COM.
Director Compensation Table
The following table sets forth information for the year ended December 31, 2015 regarding the compensation awarded to, earned by or paid to our nonemployee directors:
| Name | Fees Earned or Paid in Cash ($) | Common ($) | Option ($) | All Other Compensation(2) ($) | Total ($) | |||||||||||||||
| Firdaus J. Dastoor | $ | 4,564 | – | $ | 425,250 | – | $ | 429,814 | ||||||||||||
| Darlene Deptula-Hicks | $ | 50,000 | – | $ | 283,500 | – | $ | 333,500 | ||||||||||||
| Roman Knyazev | $ | 4,564 | – | $ | 354,375 | – | $ | 358,939 | ||||||||||||
| Dr. Roger Kornberg(4) | – | – | – | – | – | |||||||||||||||
| Artur Isaev(5) | $ | 3,378 | – | – | – | $ | 3,378 | |||||||||||||
| Dr. Timothy Cote(5) | $ | 18,750 | – | – | $ | 4,000 | $ | 22,750 | ||||||||||||
| Dr. Dmitry Genkin(5) | $ | 1,186 | $ | 1,872,259 | (3) | $ | 354,375 | $ | 72,594 | $ | 2,300,414 | |||||||||
| Mark Leuchtenberger(5) | $ | 15,385 | – | – | – | $ | 15,385 | |||||||||||||
| Jeffrey F. Eisenberg | – | – | – | – | – | |||||||||||||||
_______________
| (1) | These amounts represent fees paid for scientific services rendered to the Company during 2015 outside the scope of their duties as directors of the Board. |
| (2) | The amounts represent the aggregate grant date fair value of stock options granted during 2015. For a discussion of the assumptions and methodology used to calculate the value of our stock options, see Note 9 to our audited financial statements included elsewhere in this prospectus. |
| (3) | This amount represents the fair value of the common stock issuance during 2015 in consideration for the assignment of a U.S. provisional patent application. The valuation of this issuance is based on the methodology set forth in Note 1 to our audited consolidated financial statements in Part 8. |
| (4) | Dr. Kornberg was appointed to the Board of Directors in February 2016. |
| (5) | These nonemployee directors were members of the Board of Directors during a portion of 2015 and subsequently resigned prior to October 20, 2016. |
| (6) | Mr. Eisenberg was appointed to the Board of Directors in July 2016. |
| 104 |
Executive Compensation
Overview
Historically, our executive compensation program has reflected our growth and corporate goals and it was designed to reward the individual executive’s contribution to such growth and achievement. This section discusses the material components of the executive compensation program for our executive officers who are named in the "2015 Summary Compensation Table" below. In 2015, our "named executive officers" and their positions were as follows:
| · | M. Scott Maguire, President, Chief Executive Officer and Director; |
| · | Colin W. Hill, former Chief Financial Officer and current Director of Lipoxen Technologies Limited; and |
| · | Dr. Henry Hoppe IV, Vice President of Drug Development. |
2015 Summary Compensation Table
The following table sets forth information concerning the compensation of our named executive officers and each of our most highly compensated employees for the years ended December 31, 2015 and 2014:
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option/JSOP Awards(1) ($) | Non-Equity Incentive Plan Compensation ($) | All Other Compensation ($) | Total ($) | ||||||||||||||||
| M. Scott Maguire, Chief | 2015 | $ | 505,735 | (2) | – | – | $ | 1,984,500 | – | $ | 60,688 | $2,550,923 | ||||||||||||
| Executive Officer | 2014 | 543,840 | – | – | – | – | 65,261 | 609,101 | ||||||||||||||||
| Colin W. Hill, (former) Chief | 2015 | 148,473 | (3) | – | – | – | – | 328,121 | 476,594 | |||||||||||||||
| Financial Officer | 2014 | 253,133 | – | – | – | – | 30,376 | 283,509 | ||||||||||||||||
| Dr. Henry Hoppe IV, Vice-President | 2015 | 195,833 | – | – | 283,500 | – | 9,792 | 489,125 | ||||||||||||||||
| of Drug Development | 2014 | 200,000 | – | – | – | – | 15,833 | 215,833 | ||||||||||||||||
_____________
| (1) | The amounts represent the aggregate grant date fair value of stock options, including Joint Stock Ownership Plan (JSOP) awards, granted during each fiscal year. The valuation of stock options is based on the assumptions and methodology set forth in Note 12 to our audited consolidated financial statements included in Part 8. |
| (2) | Mr. Maguire earned $505,735 for the year ended December 31, 2015, pursuant to his written employment agreement with the Company. Of the 2015 salary amount disclosed above 50% was paid in cash and 50% was deferred and accrued pursuant to an unwritten arrangement between the Company and Mr. Maguire. |
| (3) | Collin W. Hill was the Chief Financial Officer and Principal Financial Officer through July 2015. Mr. Hill continues in the capacity of Director of Lipoxen Technologies Limited, a wholly-owned subsidiary of the Company. Mr. Hill’s 2015 “All Other Compensation” includes amounts pursuant to his separation settlement agreement with the Company. |
Narrative Disclosure to Summary Compensation Table
Base Salary
The named executive officers receive a base salary to compensate them for services rendered to us. The base salary payable to each named executive officer is intended to provide a fixed component of compensation reflecting the executive's skill set, experience, role and responsibilities. The actual base salaries paid to each named executive officer for 2015 are set forth in the 2015 Summary Compensation Table above.
Annual Cash Bonuses. Although we do not have a formal performance based cash bonus plan, our board of directors may grant annual discretionary bonuses based upon the achievement of specific individual and Company-wide performance goals. We did not grant any cash bonuses to our named executive officers during, or related to performance in fiscal year 2015.
Equity Compensation. During the year ended December 31, 2015, 242,426 stock options with an exercise price of $13.86 were granted to our named executive officers under the Xenetic Biosciences, Inc. Equity Incentive Plan.
| 105 |
We have historically granted stock options under our 2014 Equity Incentive Plan, as amended, to our directors and employees (including our named executive officers).
Option Exercises during Fiscal Year. During the year ended December 31, 2015, there were no options exercised by our named executive officers.
Other Elements of Compensation
Equity Bonus
We intend to award cash bonuses to our employees upon the consummation of this offering. The principal purpose of this is to award our employees for their services to us in general and in connection with this offering, and to retain and motivate our employees. We expect to award bonuses in the amount of fifty percent (50%) of each employee’s respective salary and have conveyed this verbally.
Employee Benefit Plans
We have a defined contribution 401(k) savings plan (401(k) Plan). The 401(k) Plan covers substantially all U.S. employees, and allows participants to defer a portion of their annual compensation on a pre-tax basis. Company contributions to the 401(k) Plan may be made at the discretion of our Board.
In the U.K., we have adopted a defined contribution plan (U.K. Plan) which qualifies under the rules established by HM Revenue & Customs. The U.K. Plan generally allows all U.K. employees to contribute a minimum of 3% of salary with no maximum limit. We contribute to the plan between 8% and 12% of the employee’s salary, depending upon seniority of the employee. We, at our discretion, may also contribute to an employee’s personal pension plan.
Employee Benefits and Perquisites
All of our full-time employees, including our named executive officers, are eligible to participate in our health and welfare plans, including medical, dental and vision benefits, medical flexible spending accounts, short-term and long-term disability insurance, and life insurance. We do not provide our named executive officers with perquisites or other personal benefits, other than the retirement, health and welfare benefits that apply uniformly to all of our employees.
No Tax Gross-Ups
We are not required to make gross-up payments to cover our named executive officers' personal income taxes that may pertain to any of the compensation or perquisites paid or provided by us.
| 106 |
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth certain information with respect to outstanding equity awards held by our named executive officers at December 31, 2015.
| Name | Grant Date | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | JSOP Awards (#) Exercisable | JSOP Awards (#) Unexercisable | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option/JSOP Expiration Date | ||||||||||||||||||||
| M. Scott Maguire | 09/06/15 | 70,707 | 141,415 | – | – | $ | 13.86 | 09/05/25 | ||||||||||||||||||||
| 06/10/10 | 15,002 | – | – | – | $ | 18.81 | 06/09/20 | |||||||||||||||||||||
| 06/10/10 | – | – | 37,402 | – | – | $ | 11.55 | NONE(1) | ||||||||||||||||||||
| 03/02/12 | – | – | 226,722 | – | – | $ | 17.49 | NONE(1) | ||||||||||||||||||||
| Colin W. Hill(2) | 06/10/10 | 15,002 | – | – | – | – | $ | 18.81 | 06/09/20 | |||||||||||||||||||
| 06/10/10 | – | – | 14,171 | – | – | $ | 11.55 | NONE(1) | ||||||||||||||||||||
| 03/02/12 | – | – | 45,590 | – | – | $ | 17.49 | NONE(1) | ||||||||||||||||||||
| Dr. Henry Hoppe IV | 09/06/15 | 30,304 | – | – | – | $ | 13.86 | 09/05/25 | ||||||||||||||||||||
| 05/01/12 | 9,697 | – | – | – | – | $ | 18.81 | 04/30/20 | ||||||||||||||||||||
| 05/01/12 | 9,697 | – | – | – | – | $ | 30.69 | 04/30/21 | ||||||||||||||||||||
| 05/01/12 | 9,697 | – | – | – | – | $ | 42.90 | 04/30/22 | ||||||||||||||||||||
__________________
| (1) | The JSOP awards do not carry an expiration date once vested. Please refer to Note 12 to our audited consolidated financial statements included in Part 8 for further description of the JSOP awards. |
| (2) | Mr. Colin Hill was the Chief Financial Officer and Principal Financial Officer through July 2015. Mr. Hill continues in the capacity of Director of Lipoxen Technologies Limited, a wholly-owned subsidiary of ours. |
Employment Agreements with our Named Executive Officers
Employment Agreement with Mr. Maguire
Mr. Maguire’s written employment agreement dated November 3, 2009 with Xenetic U.K. for a term then commencing and continuing thereafter unless and until terminated by either Mr. Maguire or the Company in writing with not less than twelve months’ notice. The employment agreement is governed by and construed in accordance with English law. Mr. Maguire’s present annual salary under his employment agreement is $505,735. The salary under this agreement is subject to periodic review by the Company without any obligation on the part of the Company to increase. The Company is required to make contributions to its Defined Contribution Pension Scheme at a rate of 12% of base salary. Additionally, Mr. Maguire is provided with life insurance coverage equal to four times base salary and is entitled to participate in the Company’s Permanent Health and Private Medical Schemes. He is also eligible to participate in the Company’s bonus and share option/equity incentive schemes in force from time to time. The agreement may be terminated by the Company for good cause without notice or payment in lieu of notice to Mr. Maguire. In case of termination upon change in control, Mr. Maguire may be entitled to twelve (12) months’ severance.
Employment Agreement with Dr. Henry Hoppe IV
Further, we also entered into a written employment agreement with Dr. Hoppe in April, 2012, which was amended in 2015. Dr. Hoppe’s current annual salary under his employment agreement is $180,000. Dr. Hoppe’s annual base salary shall be re-determined annually by the Chief Executive Officer or by the Board and he is eligible for a performance bonus of up to 25% of his then current annual base salary as provided in the agreement. If the Company terminates his agreement without cause or if he terminates his employment agreement with cause, then Dr. Hoppe shall be entitled to a severance payment equal to six months of his annual base salary, plus one month additional salary for each complete year of employment starting with the first anniversary of the effective date of the employment agreement which employment agreement was effective April 2012. The agreement may be terminated by the Company for good cause without notice or payment in lieu of notice to Dr. Hoppe.
| 107 |
Compensation Risk Assessment
We believe that although a portion of the compensation provided to our executive officers and other employees is performance-based, our executive compensation program does not encourage excessive or unnecessary risk taking. This is primarily due to the fact that our compensation programs are designed to encourage our executive officers and other employees to recognize and support both short-term and long-term strategic goals, in particular in connection with our pay-for-performance compensation philosophy. As a result, we do not believe that our compensation programs are reasonably likely to have a material adverse effect on us.
Employee Share Plans
Stock-Based Compensation Plans
Prior to the Acquisition, the Company had two incentive stock plans, the Lipoxen plc Unapproved Share Option Plan (2000 Stock Plan) and the Xenetic Biosciences plc 2007 Share Option Scheme (2007 Stock Plan). Subsequent to the Acquisition, the 2000 and 2007 Stock Plans were converted to reflect the new shares issued by the Company under the Scheme of Arrangement related to the Acquisition. As part of the conversion, option holders under the 2000 and 2007 Stock Plan have the right to subscribe for a number of shares of common stock in the Company (Replacement Option Shares) in exchange for the cancellation and surrender by the option holder of the original options granted by the 2000 and 2007 Stock Plans. The number of Replacement Option Shares is determined in the same manner in which the shareholders of Xenetic UK were given the right to acquire shares of common stock in the Company according to the Acquisition. The aggregate exercise price payable in U.S. dollars for Replacement Option Shares is the same as the aggregate exercise price in pounds sterling of the original options, using a foreign currency exchange rate for pounds sterling into U.S. dollars of 1.6531, being the rate quoted by Barclays Bank plc at 12 noon Greenwich Mean Time (GMT) on January 22, 2014, the date of the Acquisition.
The Equity Incentive Plan
The Equity Incentive Plan (2014 Plan) was adopted and became effective January 23, 2014. The purpose of the 2014 Plan is to enhance the profitability and value of the Company for the benefit of its stockholders by enabling the Company to offer all eligible present and future employees, consultants and nonemployee directors stock-based incentives in the Company in order to attract, retain and reward such individuals and strengthen the mutual interests between such individuals and the Company’s stockholders.
The 2014 Plan provides for the grant of any or a combination of incentive stock options, nonqualified stock options, restricted stock awards, or any other stock based award, including any restricted stock unit to all eligible present and future employees, consultants and nonemployee directors. The Company has broad authority to determine whether and to what extent awards are to be granted under the 2014 Plan.
The aggregate number of shares of common stock that may be issued under the 2014 Plan shall not exceed 15% of the issued and outstanding shares of common stock of the Company. Subsequent to the Acquisition, holders of awards under the 2000 Stock Plan and 2007 Stock Plan either forfeited those awards or consented in writing to convert those awards into the 2014 Plan, pursuant to a rollover deed.
Stock-Based Compensation Plan Information
The following table sets forth information as of December 31, 2015 with respect to compensation plans (including individual compensation arrangements) under which equity securities are authorized for issuance:
Number of Securities to be Issued upon Exercise of Outstanding Options, Warrants and Rights (a) | Weighted Average Exercise Price of Outstanding Options, Warrants and Rights (b) | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) (c) | ||||||||||
| Equity compensation plans approved by security holders | 688,823 | $ | 14.95 | 2,090 | ||||||||
| Equity compensation plans not approved by security holders | – | – | – | |||||||||
| Total | 688,823 | $ | 14.95 | 2,090 | ||||||||
| 108 |
Certain Relationships and Related Party Transactions
Other than compensation arrangements, we describe below the transactions and series of similar transactions to which we were a party or will be a party since January 1, 2013, in which:
| · | the amounts involved exceeded or will exceed $120,000 or one percent of the average of our total assets at year end for the last two completed fiscal years; and | |
| · | any of our directors, executive officers or holders of more than 5% of our capital stock, or any member of the immediate family of the foregoing persons, had or will have a direct or indirect material interest. |
Compensation arrangements for our directors and named executive officers are described elsewhere in this prospectus.
Policy Regarding Related Party Transactions
Our Board has adopted a written related party transaction policy to set forth the policies and procedures for the review and approval or ratification of related party transactions. Any transaction between the Company and its officers, directors, principal stockholders or affiliates are required to be on terms no less favorable to us than could be reasonably obtained in arms-length transactions with independent third-parties, and any such material related party transactions must also be reviewed and approved by a majority of the Board of Directors. All of the actions described in this section occurred prior to the adoption of this policy.
Relationship with SynBio LLC
SynBio is one of our largest shareholder and currently owns approximately 9.9% of our common stock and all of of our outstanding Series A Preferred Stock. In August 2011, we entered into a Co-Development Agreement with SynBio, which is still in effect, pursuant to which we granted an exclusive license to SynBio to develop pharmaceutical products within Russia and the CIS using certain molecule(s) based on SynBio’s technology and our proprietary technologies: PolyXen, OncoHist and ImuXen. In return, SynBio granted us an exclusive license to use the pre-clinical and clinical data generated by SynBio in certain agreed upon products and engage in the development of commercial drug candidates.
The Co-Development Agreement provides for the sale of certain research supplies between each party to the agreement. For the years ended December 31, 2015 and 2014, we did not recognized any supply service revenues from sales to SynBio in connection with the Co-Development Agreement.
On September 23, 2016, SynBio exchanged 970,000 shares of our common stock i for an equal number of shares of Series A Preferred Stock.
Loan from SynBio LLC
In May 2011, we received a short term unsecured loan facility of up to $1.7 million from SynBio, of which $152,529 was outstanding as of June 30, 2016. In connection with the Kevelt APA, we made a series of payments during the six months ended June 30, 2016 totaling $242,471 to creditors of Kevelt. Pursuant to the Kevelt APA such payments are considered direct offsets to the loan with SynBio. The loan had an interest rate of 8.04% per annum as of the date of grant, with interest payable upon repayment of the loan, which was to be seven months after the closing date of the loan. During 2012, the loan matured and the parties agreed that the loan can be called with full repayment of the outstanding principal including accrued interest upon future agreement by both parties. It was also agreed as of July 1, 2012 that no further interest on the outstanding loan balance would accrue. During 2015, we entered into a forbearance agreement with SynBio in regards to the loan which provided for the deferral of all collection efforts and waiver of any default on the loan until the earlier of the completion of a $7 million capital raise or November 15, 2016. We anticipate repaying the remainder of the loan facility, in the amount of $152,529, with proceeds from this offering.
Relationship with PJSC Pharmsynthez
PJSC Pharmsynthez (formerly, OJSC Pharmsynthez), (Pharmsynthez) currently owns 53.3% of our common stock. In November 2011, we entered into a collaborative research and development license agreement with Pharmsynthez (Pharmsynthez Arrangement) pursuant to which we granted an exclusive license to Pharmsynthez to develop, commercialize and market six product candidates based on our PolyXen and ImuXen technology in certain territories. In exchange, Pharmsynthez granted us an exclusive license to use any pre-clinical and clinical data developed by Pharmsynthez, within the scope of the Pharmsynthez Arrangement, and to engage in further research, development and commercialization of drug candidates outside of certain territories at our own expense.
| 109 |
Financing Agreements with PJSC Pharmsynthez
In July 2015, we entered into a Securities Purchase Agreement with Pharmsynthez providing for the issuance of certain promissory notes and certain warrants to purchase shares of our common stock.
In November 2015, we entered into the Kevelt APA with AS Kevelt and Pharmsynthez. The Kevelt APA provided for the transfer to us of certain intellectual property rights with respect to the immunomodulatory product candidate Virexxa held by AS Kevelt. In April 2016, we completed the purchase of the intellectual property rights associated with the Kevelt APA and issued 3,045,455 shares of common stock pursuant to the agreement.
The Kevelt APA also provided for our issuance of certain convertible promissory notes and warrants to purchase shares of our common stock. During Q1 2016, we issued $3.5 million of convertible promissory notes to Pharmsynthez receiving $3.5 million in cash proceeds. In April 2016, Pharmsynthez converted all of its convertible promissory notes (both the July 2015 note and the Q1 2016 notes) and associated interest into 1,373,036 shares of common stock. As such, we issued to Pharmsynthez 1,373,036 shares of common stock in connection with conversion of the convertible notes, which amount, together with the 3,045,455 shares of common stock in connection with the closing of the Kevelt APA, resulted in an aggregate of 4,418,491 new shares of common stock being issued to Pharmsynthez. During Q3 2016, we issued $1.0 million of convertible promissory notes to Pharmsynthez receiving $1.0 million in cash proceeds of which $0.5 million may be applied toward the Offering at the option of Pharmsynthez.
Pharmsynthez has a contractual obligation to purchase an aggregate of up to approximately $6 million in shares of our common stock and warrants in this offering at the initial public offering price and on the same terms as the other purchasers in this offering.
Pharmsynthez is a related party of SynBio, which is related party of ours. In addition, one of our directors is also a director of SynBio and Pharmsynthez.
Relationship with Serum Institute
Serum Institute currently owns approximately 9.9% of our common stock. In the period from 2004 through 2011, we entered into and amended certain license and supply agreements with Serum Institute. The original license agreement with Serum Institute was a collaborative Development and Manufacturing Arrangement (DMA) to develop agreed upon potential commercial product candidates using our PolyXen technology. Following the 2011 amendment, which is still in effect, Serum Institute retained an exclusive license to use our PolyXen technology to research and develop one potential commercial product, Polysialylated Erythropoietin (PSA-EPO) in territories excluding the United States of America, European Economic Area, Japan, Russia, the CIS, South Korea and other certain territories. Serum Institute will be responsible for conducting all pre-clinical and clinical trials required to achieve regulatory approvals within the certain predetermined territories at Serum Institute’s own expense.
Manufacturing Agreement with Serum Institute
The 2011 amendment with Serum Institute also provides for the supply of PSA by Serum Institute to us and our collaborative partners. Serum Institute has the non-exclusive right to supply PSA to us, and our collaborative partners and customers on a cost-plus basis. On an individual basis, Serum Institute may enter into separate supply agreements with us and/or our collaborative partners for the purpose of providing a supply of PSA directly to the collaborative partners. Further, any agreement between Serum Institute and a collaborative partner shall not create any obligation or liability for us.
During 2015 and 2014, we paid Serum Institute $115,000 and zero, respectively, for the supply of PSA or PSA-EPO for use in our ErepoXen human clinical trials being conducted in Australia.
Relationship with Shire plc (formerly Baxalta and Baxter Healthcare SA)
As of June 30, 2016, Shire owned approximately 4.2% of our common stock. We have entered into an exclusive research, development, license and supply agreement with Baxter SA and Baxter Healthcare Corporation (together referred to as Baxter) to develop products using the ours and Shire’s proprietary technologies. The agreement with Shire was originally entered into in August 2005 and has been amended several times, most recently in January 2014. The 2014 amendment resulted in increased development, regulatory, sales and deadline extension receipts, restructured target deadlines and royalty receipts on potential net sales.
| 110 |
Shire’s $10 Million Equity Investment
In connection with the January 2014 amendment, we entered into a stock purchase agreement with Shire (initially entered into with Baxter SA, and subsequently transferred to Baxalta), pursuant to which we sold to Shire 324,097 shares of our common stock, par value $0.001 per share (Shares) for $10 million. Pursuant to the stock purchase agreement, Shire agreed that until the earlier of (i) three months after the effective date of a listing of our common stock on the NASDAQ Stock Market or (ii) January 29, 2015 (such earlier date, the Lock-Up Expiration Date), Shire would not assign, transfer, sell or dispose of the Shares to any party other than a wholly owned subsidiary. In addition, Shire agreed that until the 12 month anniversary of the Lock-Up Expiration Date, it would not sell or offer to sell any shares of our common stock in an amount that would exceed 15% of the daily trading volume of Company’s common stock on the principal market or exchange on which the shares of Company’s common stock are traded, and in no event would Shire sell or offer to sell more than 15% of the Shares in any one month period. In October 2015, Shire agreed in writing not to sell any of its shares currently held before June 30, 2016 and then further to limit the sale of its shares for an additional six months (to December 2016) to a price of no less than $41.25 per share.
Consulting Services Agreement with Dr. Dmitry Genkin
Dr. Dmitry Genkin is party to a letter agreement with the Company under which he is entitled to an annual fee of £750 paid in quarterly installments for his services as Director of the Company. During 2015, Dr. Genkin charged the Company approximately $1,000 in respect of director services provided. In addition, Dr. Genkin provides research and consulting services to the Company, for which he was paid by the Company approximately $73,000 during the year ended December 31, 2015.
Consulting Services Agreement with Dr. Timothy R. Coté
Dr. Timothy R. Coté is party to a letter agreement with the Company under which he is entitled to an annual fee of $25,000 paid in quarterly installments for his services as Director of the Company. In addition his agreement provides for payment of an additional annual fee of between $3,000 and $10,000 as compensation for attendance at up to four board meetings per year plus issuance of options to purchase up to 1,516 shares of our common stock, subject to certain vesting requirements.
Under his agreement, Dr. Coté’s consulting firm, Coté Orphan Drug Consulting, LLC (CODC), shall have the exclusive right to advise the Company on all orphan drug filings with the U.S. Food and Drug Administration for so long as Dr. Coté remains a member of the Board of Directors. During 2015, Dr. Coté charged the Company $18,750 in respect of director services provided and $4,000 for advisory services related to certain orphan drug filings.
Deferred Salary Arrangement with Mr. M. Scott Maguire
Mr. M. Scott Maguire is our Chief Executive Officer. Mr. Maguire current annual salary is $505,735 pursuant to his written employment agreement with the Company. Of Mr. Maguire’s 2015 salary amount and 2016 salary amount through today, fifty percent (50%) has been paid in cash and fifty percent (50%) has been deferred and accrued pursuant to an unwritten arrangement between us and Mr. Maguire. In July 2016, we issued a convertible promissory note in the amount of $369,958 and warrants to purchase 37,369 shares of our common stock at $6.60 per share to Mr. Maguire for the deferred salary. We also entered into a Deferred Salary Security Agreement with Mr. Maguire, pursuant to which Mr. Maguire agreed to continue to defer fifty percent (50%) of his salary until the earlier to occur of: (i) the closing of a public offering of our securities concurrent to a NASDAQ listing, or (ii) September 30, 2016 (the “Deferral End Date”). All deferred salary shall become due and payable on the Deferral End Date. As security for the payment of the deferred salary, we granted Mr. Maguire a continuing security interest in our assets, including all inventory, accounts, accounts receivable, equipment, trademarks, contracts, copyrights and general intangibles, provided, however, that pursuant to a Debt Subordination Agreement by and between Mr. Maguire and Pharmsynthez, the payment of such deferred salary is subordinate to the prior payment in full in cash of any and all of our indebtedness and liabilities to Pharmsynthez.
Indemnification Agreements
We have entered into agreements to indemnify our directors and executive officers to the maximum extent allowed under Nevada law. Subject to the provisions of these agreements, these agreements, among other things, provide for indemnification of these individuals for certain expenses (including attorneys’ fees), judgments, fines and settlement amounts reasonably incurred by such person in any action or proceeding, including any action by or in our right, on account of any services undertaken by such person on behalf of us or that person’s status as a member of our Board.
| 111 |
Principal Stockholders
The following table and footnotes set forth certain information known to us regarding beneficial ownership of our capital stock as of October 20, 2016, for:
| · | each person known by us to be the beneficial owner of more than 5% of our capital stock; | |
| · | our named executive officers; | |
| · | each of our directors; and | |
| · | all executive officers and directors as a group. |
The number of shares beneficially owned by each entity, person, director or executive officer is determined in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares over which the individual has sole or shared voting power or investment power as well as any shares that the individual has the right to acquire within 60 days through the exercise of any stock option, warrants or other rights. Except as otherwise indicated, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of common stock held by that person or entity.
The percentage of shares beneficially owned is computed on the basis of 8,288,643 shares of our common stock outstanding as of October 20, 2016, on an as-converted basis. Shares of our common stock that a person has the right to acquire within 60 days after October 20, 2016 are deemed outstanding for purposes of computing the percentage ownership of the person or entity holding such rights, but are not deemed outstanding for purposes of computing the percentage ownership of any other person, except with respect to the percentage ownership of all directors and executive officers as a group. Unless otherwise indicated below, the address for each beneficial owner listed is c/o Xenetic Biosciences, Inc., at 99 Hayden Ave, Suite 230, Lexington, Massachusetts 02421.
| Name of Beneficial Owner | Number
of Shares Beneficially Owned |
Percentage
Beneficially Owned | |
| Named Executive Officers and Directors | |||
| M. Scott Maguire | 531,863 | (1) | 6.1% |
| Firdaus J. Dastoor | 78,788 | (2) | * |
| Darlene Deptula-Hicks | 21,416 | (3) | * |
| Roman Knyazev(4) | 25,253 | (5) | * |
| Dr. Roger Kornberg | - | * | |
| Jeffrey F. Eisenberg | - | * | |
| All executive officers and directors as a group | 657,320 | (6) | 7.4% |
| 5% Stockholders | |||
| PJSC Pharmsynthez(4) | 5,428,594 | (7) | 58.4% |
| SynBio LLC | 821,567 | (8) | 9.9% |
| Shire plc | 534,171 | (9) | 6.3% |
| Serum Institute of India Private Limited | 842,519 | (10) | 9.9% |
| OPKO Health, Inc.(12) | 309,215 | (13) | 3.7% |
| * | Represents beneficial ownership of less than one percent (1%). |
| (1) | The total beneficial ownership consists of 73,953 shares of common stock owned directly or through nominee trusts, 264,124 JSOP award shares and 193,786 shares issuable upon exercise of warrants and options that are exercisable within 60 days of October 20, 2016. |
| (2) | The total beneficial ownership consists of 78,788 shares issuable upon exercise of warrants and options that are exercisable within 60 days of October 20, 2016. |
| (3) | The total beneficial ownership consists of 21,416 shares issuable upon exercise of options that are exercisable within 60 days of October 20, 2016. |
| (4) | Mr. Knyazev is the Deputy Chairman of the Board of Directors of Pharmsynthez. |
| (5) | The total beneficial ownership consists of 25,253 shares issuable upon exercise of options that are exercisable within 60 days of October 20, 2016. |
| 112 |
| (6) | The total beneficial ownership consists of 73,953 shares of common stock owned directly or through nominee trusts, 264,124 JSOP award shares, and 319,243 shares issuable upon exercise of warrants and options that are exercisable within 60 days of October 20, 2016. |
| (7) | The total beneficial ownership consists of 4,418,491 shares of common stock owned directly and 1,010,103 shares issuable upon exercise of warrants that are exercisable within 60 days of October 20, 2016. The address of PJSC Pharmsynthez is Office Center IT Park, 25 Liter ZH, Krasnogo Kursanta ul., St. Petersburg, 197110, Russia. |
| (8) | The total beneficial ownership consists of 821,567 shares of common stock owned directly including 145,455 held in escrow. The address of SynBio LLC is Building 2, 55/1, Leninsky Prospekt, Moscow, Russian Federation. |
| (9) | The total beneficial ownership consists of 395,131 shares of common stock owned directly and 139,040 shares issuable upon exercise of warrants that are exercisable within 60 days of October 20, 2016. The address of Shire plc U.S. headquarters is 300 Shire Way, Lexington, MA 02421. |
| (10) | The total beneficial ownership consists of 630,396 shares of common stock owned directly and indirectly by related affiliates of Serum Institute of India Private Limited and 212,123 shares issuable upon exercise of warrants that are exercisable within 60 days of October 20, 2016. The address of Serum Institute of India is S. No. 212/2, Off Soli Poonawalla Road, Hadapsar, Pune, 411028, Maharashtra, India. |
| (12) | OPKO Health in 2013 acquired a significant ownership position in Pharmsynthez. By virtue of such relationship, OPKO Health may be deemed to have voting and investment power with respect to the shares held by Pharmsynthez noted above and as a result may be deemed to have beneficial ownership over such shares. |
| (13) | The total beneficial ownership consists of 309,215 shares of common stock owned directly. The address of OPKO Health Inc. is 4400 Biscayne Boulevard, Miami, FL 33137. |
| 113 |
Description of Capital Stock
The following summary describes our capital stock and the material provisions of our amended and restated certificate of incorporation and our amended and restated bylaws, which will become effective immediately prior to the consummation of this offering. Because the following is only a summary, it does not contain all of the information that may be important to you. For a complete description, you should refer to our amended and restated certificate of incorporation and amended and restated bylaws, copies of which have been filed as exhibits to the registration statement of which this prospectus is part.
General
Immediately prior to the consummation of this offering, we will file our amended and restated certificate of incorporation that authorizes capital stock of 45,454,546 shares of common stock, par value $0.001 per share, and 10,000,000 shares of preferred stock, par value $0.001 per share, 1,000,000 of which are designated as Series A Preferred Stock, 2,500,000 of which are anticipated to be designated as Series B Preferred Stock, and 6,500,000 of which shares of preferred stock are undesignated.
As of June 30, 2016, there were outstanding:
| · | 9,024,872 shares of our common stock, on an as-converted basis, held by approximately 427 stockholders of record. |
| · | 671,853 shares of our common stock issuable upon exercise of stock options, at a weighted average exercise price of $14.99 per share. |
| · | 681,878 shares of common stock reserved for future issuance under our 2014 Plan. |
Common Stock
Voting Rights
Our common stock is entitled to one vote per share on all matters submitted to a vote of the stockholders, including the election of directors. Except as otherwise required by law or provided in any resolution adopted by our board of directors with respect to any series of preferred stock, the holders of our common stock will possess all voting power. Generally, all matters to be voted on by stockholders must be approved by a majority (or, in the case of election of directors, by a plurality) of the votes entitled to be cast by all shares of our common stock that are present in person or represented by proxy, subject to any voting rights granted to holders of any preferred stock. Our stockholders do not have cumulative rights in the election of directors. Holders of our common stock representing 50% of our capital stock issued, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum at any meeting of our stockholders. A vote by the holders of a majority of our outstanding shares is required to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to our articles of incorporation. Our articles of incorporation do not provide for cumulative voting in the election of directors.
Dividends
Subject to any preferential rights of any outstanding series of preferred stock created by our board of directors from time to time, the holders of shares of our common stock will be entitled to such cash dividends, non-cumulative, as may be declared from time to time by our board of directors from funds available therefore.
Liquidation
Subject to any preferential rights of any outstanding series of preferred stock created from time to time by our board of directors, upon liquidation, dissolution or winding up, the holders of shares of our common stock will be entitled to receive pro rata all assets available for distribution to such holders.
| 114 |
Rights and Preferences
In the event of any merger or consolidation with or into another company in connection with which shares of our common stock are converted into or exchangeable for shares of stock, other securities or property (including cash), all holders of our common stock will be entitled to receive the same kind and amount of shares of stock and other securities and property (including cash). Holders of our common stock have no pre-emptive, conversion, subscription or other rights and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of our preferred stock that we may designate in the future.
Fully Paid and Nonassessable
All of our outstanding shares of common stock are, and the shares of common stock to be issued in this offering will be, fully paid and nonassessable.
Preferred Stock
Our board of directors, the Board, has the authority, without further action by our stockholders, to issue up to 10,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption or repurchase, liquidation preferences, sinking fund terms and the number of shares constituting, or the designation of, such series, any or all of which may be greater than the rights of our common stock. The issuance of our preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon a liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change of control of the Company or other corporate action.
Series A Preferred Stock
Our Board has designated 1,000,000 shares of preferred stock as Series A Preferred Stock, $0.001 par value. As of October 20, 2016, there were 970,000 shares of Series A preferred stock outstanding. Although there is no current intent to do so, except for Series B Preferred Stock, our Board may, without stockholder approval, issue shares of an additional class or series of preferred stock with voting and conversion rights which could adversely affect the voting power of the holders of the common stock or the convertible preferred stock, except as prohibited by the certificate of designation of preferences, rights and limitations of Series A preferred stock.
The following is a summary of the material terms of our Series A preferred stock. For more information, please refer to the certificate of designation of Series A preferred stock filed as an exhibit to the registration statement of which this prospectus is a part.
Liquidation. Upon any dissolution, liquidation or winding up, whether voluntary or involuntary, holders of Series A preferred stock will be entitled to receive distributions out of our assets, of an amount equal to $4.80 per share of Series A preferred stock (as adjusted for stock splits, combinations, reorganizations and the like) plus any accrued and unpaid dividends thereon before any distributions shall be made on the common stock or any series of preferred stock ranked junior to the Series A preferred stock.
Dividends. Holders of the Series A preferred stock are entitled to receive a non-cumulative, annual cash dividend of $0.24 per share of Series A Preferred Stock, when and if declared by our Board, out of our assets legally available therefore. Dividends shall not be cumulative. No dividends or other distribution will be made on the common stock or any series of preferred stock ranked junior to the Series A preferred stock unless the dividend on the Series A Preferred Stock has been paid current and a reserve has been made for the next calendar year.
Conversion. Each share of Series A preferred stock is convertible, at any time and from time to time at the option of the holder thereof, with a minimum of 61 days’ advance notice to the Company, into one share of common stock.
| 115 |
Conversion Price Adjustment.
Stock Dividends and Stock Splits. If we pay a stock dividend or otherwise make a distribution payable in shares of common stock on shares of common stock or any other common stock equivalents, subdivide or combine outstanding common stock, or reclassify common stock, the conversion rate will be adjusted to match the conversion rate immediately before such event.
Fundamental Transaction. If we effect a reorganization, undergo a change in control event, or enter into any plan or arrangement contemplating our dissolution, then upon any subsequent conversion of Series A preferred stock, the holder thereof shall have the right to receive, for each share of common stock that would have been issuable upon such conversion immediately prior to the occurrence of such transaction, the number of shares of the successor's or acquiring corporation's common stock or of our common stock, if we are the surviving corporation, and any additional consideration receivable as a result of such transaction by a holder of the number of shares of common stock into which Series A preferred stock is convertible immediately prior to such transaction. A change in control event means a sale of all or substantially all of our assets or an acquisition of the Company by another entity by means of any transaction or series of related transactions (including, without limitation, a reorganization, consolidated or merger) that results in the transfer of fifty percent (50%) or more of the outstanding voting power of the Company.
Voting Rights. The holders of Series A preferred stock shall be entitled to vote on any matters on which the common stock shall be entitled to vote. The holders of Series A preferred stock vote together with common stock holders on a 1:1 basis with respect to matters that generally require the approval of holders of common stock. The holders of Series A preferred stock have voting rights as to proposals that specifically affect their shares by law, in which they will vote separately and the vote necessary to approve such proposals will be as set by law.
Fractional Shares. No fractional shares of common stock will be issued upon conversion of Series A preferred stock. Rather, we shall round up to the next whole share.
Redemption. At any time after December 31, 2016, upon 30 days prior written notice, we may require the holder of any Series A Preferred Stock to convert any or all of such holder’s Series A preferred stock to common stock at a rate of one share of Series A Preferred Stock to one share of common stock.
Series B Preferred Stock
We anticipate our Board will designate 2,500,000 shares of preferred stock as Series B Preferred Stock, $0.001 par value. As of October 20, 2016, there were zero shares of Series B Preferred Stock outstanding. Although there is no current intent to do so, our Board may, without stockholder approval, issue shares of an additional class or series of preferred stock with voting and conversion rights which could adversely affect the voting power of the holders of the common stock or the convertible preferred stock, except as prohibited by the certificate of designation of preferences, rights and limitations of Series B Preferred Stock.
The following is a summary of the anticipated material terms of our Series B Preferred Stock.
Liquidation. Upon any dissolution, liquidation or winding up, whether voluntary or involuntary, holders of Series B Preferred Stock will be entitled to receive distributions out of our assets, of an amount equal to $ per share of Series B Preferred Stock (as adjusted for stock splits, combinations, reorganizations and the like) plus any accrued and unpaid dividends thereon before any distributions shall be made on the common stock or any series of preferred stock ranked junior to the Series B Preferred Stock, which includes Series A Preferred Stock.
Dividends. Subject to any preferential rights of any outstanding series of preferred stock created by our Board from time to time, the holders of shares of our common stock will be entitled to such cash dividends, non-cumulative, as may be declared from time to time by our Board from funds available therefore.
Conversion. Each share of Series B Preferred Stock is convertible, at any time and from time to time at the option of the holder thereof, into one share of common stock, subject to the adjustments described below.
| 116 |
Conversion Price Adjustment
Stock Dividends and Stock Splits. If we pay a stock dividend or otherwise make a distribution payable in shares of common stock on shares of common stock or any other common stock equivalents, subdivide or combine outstanding common stock, or reclassify common stock, the conversion rate will be adjusted to match the conversion rate immediately before such event.
Fundamental Transaction. If we effect a reorganization, undergo a change in control event, or enter into any plan or arrangement contemplating our dissolution, then upon any subsequent conversion of Series A preferred stock, the holder thereof shall have the right to receive, for each share of common stock that would have been issuable upon such conversion immediately prior to the occurrence of such transaction, the number of shares of the successor's or acquiring corporation's common stock or of our common stock, if we are the surviving corporation, and any additional consideration receivable as a result of such transaction by a holder of the number of shares of common stock into which Series A preferred stock is convertible immediately prior to such transaction. A change in control event means a sale of all or substantially all of our assets or an acquisition of the Company by another entity by means of any transaction or series of related transactions (including, without limitation, a reorganization, consolidated or merger) that results in the transfer of fifty percent (50%) or more of the outstanding voting power of the Company.
Subsequent Equity Sales. The Series B Preferred Stock has full ratchet price based anti-dilution protection, subject to customary carve outs, in the event of a down-round financing at a price per share below the stated value of the Series B Preferred Stock.
Voting Rights. Except as otherwise provided in the Series B Preferred Stock certificate of designation or required by law, the Series B Preferred Stock has no voting rights. The holders of Series B Preferred Stock have voting rights as to proposals that specifically affect their shares by law, in which they will vote separately and the vote necessary to approve such proposals will be as set by law.
Fractional Shares. No fractional shares of common stock will be issued upon conversion of Series B Preferred Stock. Rather, we shall round up to the next whole share.
Warrants
Warrants to be Issued as Part of this Offering
The warrants offered in this offering will be issued in a form filed as an exhibit to the registration statement of which this prospectus is a part. You should review a copy of the form of warrant for a complete description of the terms and conditions applicable to the warrants. The following is a brief summary of the warrants and is subject in all respects to the provisions contained in the form of warrant.
Duration and Exercise Price
Each warrant offered hereby will have an exercise price of $ per share. The Class A warrants will be exercisable six months after issuance. The Class B Warrants will be immediately exercisable. The warrants will expire on the fifth anniversary of the original issuance date.
Exercisability
The warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the warrant to the extent that the holder would own more than 4.99% of the outstanding common stock after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants.
| 117 |
Cashless Exercise
If, at the time a holder exercises its warrant, there is no effective registration statement registering, or the prospectus contained therein is not available for an issuance of the shares underlying the warrant to the holder, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of our common stock determined according to a formula set forth in the warrant.
Certain Adjustments
The warrant provides that the exercise price is subject to adjustment in the event of stock splits, reverse stock splits and similar events.
Fundamental Transactions
In the event of any fundamental transaction, as described in the warrants and generally including any merger with or into another entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our common stock, the holder will have the right to have such warrants and all obligations and rights thereunder assumed by the successor or acquiring corporation. In the event of a fundamental transaction involving an all cash transaction, a Rule 13e-3 transaction, or an entity not traded on a national securities exchange, the holder of a warrant may, up until 30 days following the fundamental transaction, require us or the successor or acquiring corporation to purchase the warrant by paying the Black Scholes Value of the remaining unexercised portion of the warrant as of the date of the fundamental transaction. For purposes of the warrant, “Black Scholes Value” means the value of the warrant based on the Black and Scholes Option Pricing Model obtained from the “OV” function on Bloomberg, L.P. (“Bloomberg”) determined as of the day of consummation of the applicable fundamental transaction for pricing purposes and reflecting (A) a risk-free interest rate corresponding to the U.S. Treasury rate for a period equal to the time between the date of the public announcement of the applicable fundamental transaction and the expiration date of the warrant, (B) an expected volatility equal to the greater of 100% and the 100 day volatility obtained from the HVT function on Bloomberg as of the trading day immediately following the public announcement of the applicable fundamental transaction, (C) the underlying price per share used in such calculation shall be the sum of the price per share being offered in cash, if any, plus the value of any non-cash consideration, if any, being offered in such fundamental transaction and (D) a remaining option time equal to the time between the date of the public announcement of the applicable fundamental transaction and the expiration date of the warrant.
Transferability
Subject to applicable laws and the restriction on transfer set forth in the warrant, the warrant may be transferred at the option of the holder upon surrender of the warrant to us together with the appropriate instruments of transfer.
No Listing
There is no established trading market for the warrants and we do not expect a market to develop. In addition, we do not intend to apply for the listing of the warrants on any national securities exchange or other trading market. Without an active trading market, the liquidity of the warrants will be limited.
Right as a Stockholder
Except as otherwise provided in the warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their warrants.
Waivers and Amendments
Subject to certain exceptions, any term of a warrant may be amended or waived with our written consent and the written consent the holder.
| 118 |
Registration Rights
Under our registration rights agreement, as amended, the holders of approximately 303 million shares of common stock, on an as-converted basis, or their transferees, have the right to require us to register their shares under the Securities Act so that those shares may be publicly resold, or to include their shares in any registration statement we file, in each case as described below.
Demand Registration Rights
The holders of approximately 9.3 million shares of our common stock, on an as-converted basis, or their transferees, are entitled to certain demand registration rights. The holders of at least twenty percent (20%) of these shares can request that we register all or a portion of their shares.
Piggyback Registration Rights
In the event that we determine to register any of our securities under the Securities Act (subject to certain exceptions), in another offering, either for our own account or for the account of other security holders, the holders of approximately 9.3 million shares of our common stock (on an as converted basis), or their transferees, will be entitled to certain "piggyback" registration rights allowing holders to include their shares in such registration, provided, however, that we shall not be required to register any securities that are eligible for resale pursuant to Rule 144, promulgated under the Securities Act, or that are the subject of a then effective registration statement. As a result, whenever we propose to file a registration statement under the Securities Act, other than with respect to a registration related to employee benefit plans, or corporate reorganizations or certain other transactions, the holders of these shares are entitled to notice of the registration and have the right, subject to limitations that the underwriters may impose on the number of shares included in the registration, to include their shares in the registration. In an underwritten offering, we are not required to include any such “piggyback” securities unless the holder thereof accepts the terms of the underwriting as agreed upon between the Company and the underwriters selected by it. Further, the underwriters have the right, subject to specified conditions and limitations, to limit the number of shares such holders may include.
Expenses of Registration
We will pay the registration expenses of the holders of the shares registered pursuant to the demand and piggyback registration rights described above.
Expiration of Registration Rights
The demand and piggyback registration rights described above will expire six months after such stockholder can sell all of its shares under Rule 144 of the Securities Act (without volume restriction or the requirement for the Company to be in compliance with the current public information required under Section c(1) of Rule 144 of the Securities Act).
| 119 |
Anti-takeover Effects of Our Articles of Incorporation, Bylaws and Nevada Law
Certain provisions of Nevada law, and our articles of incorporation and our bylaws that will become effective immediately prior to the consummation of this offering contain provisions that could make the following transactions more difficult: acquisition of us by means of a tender offer; acquisition of us by means of a proxy contest or otherwise; or removal of our incumbent officers and directors. It is possible that these provisions could make it more difficult to accomplish or could deter transactions that stockholders may otherwise consider to be in their best interest or in our best interests, including transactions that might result in a premium over the market price for our shares.
These provisions, summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Nevada Anti-Takeover Laws
We are subject to Section 78.438 of the Nevada Revised Statutes, unless we expressly elect in the articles of incorporation not to be governed by this Section. The section prohibits persons deemed "interested stockholders" from engaging in a "combination" with a publicly-held Nevada corporation for two years following the date these persons become interested stockholders unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. In general, an "interested stockholder" is a person who, beneficial owns, directly or indirectly, 10 percent or more of the voting power of the outstanding voting shares of the Company; or an affiliate or associate of the resident domestic corporation who at any time within 2 years immediately before the date in question was the beneficial owner, directly or indirectly, of 10 percent or more of the voting power of the then outstanding shares of the Company. In general, a "combination" includes a merger, asset or stock sale, or other transaction with the interested stockholder. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by the board of directors, such as discouraging takeover attempts that might result in a premium over the market price of our common stock. Our articles of incorporation and bylaws do not state that these provisions do not apply. However, the statute is limited to corporations that are organized in the state of Nevada and that have 200 or more stockholders, at least 100 of whom are stockholders of record and residents of the State of Nevada; and does business in the State of Nevada directly or through an affiliated corporation. Because of these conditions, the statute as of July 7, 2016, currently does not apply to our Company.
Undesignated Preferred Stock
The ability to authorize undesignated preferred stock pursuant to our amended and restated certificate of incorporation will make it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of us. These and other provisions may have the effect of deterring hostile takeovers or delaying changes in control or management of the Company.
Exchange Listing
Our common stock is quoted on the OTCQB under the trading symbol “XBIO.” We have applied to have our common stock listed on The NASDAQ Capital Market under the symbol “XBIO.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Empire Stock Transfer, Inc. The transfer agent and registrar’s address is 1859 Whitney Mesa Drive, Henderson, Nevada 89014, and its telephone number is 702-818-5898.
| 120 |
Certain material U.S. Federal Income Tax Considerations
The following is a summary of certain material U.S. federal income tax considerations relating to the acquisition, ownership and disposition of shares of our Series B Preferred Stock and warrants issued pursuant to this offering. This summary deals only with shares of our Series B Preferred Stock to purchase common stock and warrants acquired by a stockholder in this offering and that are held as capital assets within the meaning of Section 1221 of the Internal Revenue Code of 1986, as amended (the “Code”). This summary does not address the U.S. federal income tax considerations applicable to a stockholder that is subject to special treatment under U.S. federal income tax laws, including: a dealer in securities or currencies; a financial institution; a regulated investment company; a real estate investment trust; a tax-exempt organization; an insurance company; a person holding our common stock as part of a hedging, integrated, conversion or straddle transaction or a person deemed to sell our common stock under the constructive sale provisions of the Code; a trader in securities that has elected the mark-to-market method of accounting; an entity that is treated as a partnership for U.S. federal income tax purposes; a person that received our common stock in connection with services provided to the company or any of its affiliates; a U.S. person whose “functional currency” is not the U.S. dollar; a “controlled foreign corporation”; a “passive foreign investment company”; or a U.S. expatriate.
This summary is based upon provisions of the Code, and applicable Treasury regulations promulgated or proposed thereunder, rulings and judicial decisions, all as in effect as of the date hereof. Those authorities may be changed, perhaps with retroactive effect, or may be subject to differing interpretations, which could result in U.S. federal income tax consequences different from those discussed below. This summary does not address all aspects of U.S. federal income tax, does not address all tax considerations that may be relevant to holders of our Series B Preferred Stock or warrants to purchase common stock in light of their personal circumstances and does not address any state, local, foreign, gift, estate or alternative minimum tax considerations.
For purposes of this discussion, a “U.S. holder” is a beneficial holder of our Series B Preferred Stock or warrants to purchase common stock that is: an individual citizen or resident of the United States for U.S. federal income tax purposes; a corporation (or any other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia; an estate the income of which is subject to U.S. federal income taxation regardless of its source; or a trust if it (1) is subject to the primary supervision of a court within the United States and one or more U.S. persons (as defined in the Code) have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
For purposes of this discussion, a “non-U.S. holder” is a beneficial holder of our Series B Preferred Stock or warrants to purchase common stock that is for U.S. federal income tax purposes an individual, corporation, estate or trust and is not a U.S. holder.
If a partnership (or an entity or arrangement that is treated as a partnership for U.S. federal income tax purposes) holds our Series B Preferred Stock or warrants to purchase common stock, the tax treatment of a person treated as a partner in the partnership for U.S. federal income tax purposes generally will depend upon the status of the partner and the activities of the partnership. Partnerships and other entities that are treated as partnerships for U.S. federal income tax purposes and persons holding our Series B Preferred Stock or warrants to purchase common stock through a partnership or other entity treated as a partnership for U.S. federal income tax purposes are urged to consult their own tax advisors.
This summary is for general information only and is not intended to be tax advice. Investors in this offering are urged to consult their own tax advisors concerning the tax considerations related to the acquisition, ownership and disposition of shares of our Series B Preferred Stock and warrants issued pursuant to this offering in light of their particular circumstances, as well as any tax considerations arising under the laws of any other jurisdiction, including any state, local and foreign income and other tax laws.
| 121 |
U.S. Holders
The following discussion is a summary of certain U.S. federal income tax considerations relevant to a U.S. holder of our Series B Preferred Stock or warrants to purchase common stock.
Distributions
Distributions with respect to our Series B Preferred Stock or common stock, if any, generally will be includible in the gross income of a U.S. holder as ordinary dividend income to the extent of our current or accumulated earnings and profits, as determined for U.S. federal income tax purposes. Any portion of a distribution in excess of current and accumulated earnings and profits will be treated as a non-taxable return of capital, up to the U.S. holder’s adjusted tax basis in its shares of our Series B Preferred Stock or common stock with respect to which the distribution was made. Any such distribution in excess of the U.S. holder’s adjusted tax basis in its shares will be treated as capital gain and as long-term capital gain if the U.S. holder’s holding period exceeds one year. If certain requirements are met (including certain holding period requirements), distributions constituting dividends paid to non-corporate U.S. holders generally will qualify for the reduced tax rate on qualified dividend income.
Distributions constituting dividends for U.S. federal income tax purposes that are paid to U.S. holders that are corporations may qualify for the 70% dividends received deduction (DRD), which is generally available to corporations that own less than 20% of the voting power or value of the outstanding stock of the distributing corporation. A U.S. holder that is a corporation holding 20% or more of the distributing corporation (by vote and value) may be eligible for an 80% DRD with respect to any such dividends. No assurance can be given that we will have sufficient earnings and profits (as determined for U.S. federal income tax purposes) to cause any distributions to be treated as dividends eligible for a DRD. In addition, a DRD is available only if certain other requirements (including certain holding period requirements) are satisfied, and a DRD may be subject to limitations in certain circumstances, which are not discussed herein.
Sale, Exchange, Redemption or Certain Other Taxable Dispositions of Our Securities
A U.S. holder of our Series B Preferred Stock or common stock generally will recognize gain or loss on the taxable sale, exchange, redemption (provided the redemption is treated as a sale or exchange), or other taxable disposition of such shares in an amount equal to the difference between such U.S. holder’s amount realized on such disposition and such U.S. holder’s adjusted tax basis in its shares sold or exchanged. A U.S. holder’s amount realized generally will equal the amount of cash and the fair market value of any property received in consideration for the shares sold or exchanged. Such gain or loss will be capital gain or loss, and will be long-term capital gain or loss if the U.S. holder’s holding period for the shares of our capital stock disposed of exceeds one year at the time of disposition. The deductibility of capital losses is subject to certain limitations. U.S. holders should consult their tax advisors regarding the treatment of capital gains and capital losses.
Purchase of Units
For U.S. federal income tax purposes, the purchase of a unit by U.S. holders will be treated as the purchase of two components: a component consisting of one share of our Series B Preferred Stock and a component consisting of one warrant to purchase one share of our common stock. The purchase price for each unit will be allocated between its two components in proportion to the relative fair market value of each at the time the unit is purchased by the holder. This allocation of the purchase price for each unit will establish a holder’s initial tax basis for U.S. federal income tax purposes in the shares and warrants that comprise each unit.
Exercise of Warrants
A U.S. holder generally will not recognize gain or loss on the exercise of a warrant and related receipt of our shares of our common stock (unless cash is received in lieu of the issuance of a fractional share of our common stock). A U.S. holder’s initial tax basis in the shares of our common stock received upon the exercise of a warrant will be equal to the sum of (a) the U.S. holder’s tax basis in such warrant plus (b) the exercise price paid by the U.S. holder on the exercise of such warrant. A U.S. holder’s holding period for the shares of our common stock received upon the exercise of a warrant will begin on the date that such warrant is exercised by the U.S. holder.
In certain limited circumstances, a U.S. holder may be permitted to undertake a cashless exercise of warrants into shares of our common stock. The U.S. federal income tax treatment of a cashless exercise of warrants into shares of common stock is unclear, and the tax consequences of a cashless exercise could differ from the consequences upon the exercise of a warrant described in the preceding paragraph. U.S. holders should consult their own tax advisors regarding the U.S. federal income tax consequences of a cashless exercise of warrants.
| 122 |
Certain Adjustments to the Warrants
An adjustment to the number of shares of our common stock that will be issued upon the exercise of a warrant, or an adjustment to the exercise price of a warrants, may be treated as a constructive distribution to a U.S. holder of the warrants if, and to the extent that, such adjustment has the effect of increasing the U.S. holder’s proportionate interest in our “earnings and profits” or assets, depending on the circumstances of such adjustment (for example, if the adjustment is to compensate for a distribution of cash or other property to our shareholders). Adjustments to the exercise price of warrants made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the holders of the warrants generally should not be considered to result in a constructive distribution. Any such constructive distribution would be taxable regardless whether there is an actual distribution of cash or other property. See the more detailed discussion of the rules applicable to distributions made by us under the heading “Distributions” above.
Conversion of Series B Preferred Stock
A U.S. holder generally will not recognize gain or loss upon the conversion of a share of Series B Preferred Stock into common stock (except in the event cash is issued in respect of fractional shares). A U.S. holder’s initial tax basis in the shares of our common stock received upon the conversion of a share of Series B Preferred Stock will be equal to the U.S. holder’s tax basis in the share of Series B Preferred Stock. A U.S. holder’s holding period for the shares of our common stock received upon the conversion of a share of Series B Preferred Stock will include the U.S. holder’s holding period in the share of Series B Preferred Stock.
Medicare Tax on Net Investment Income
An additional 3.8% Medicare tax will be imposed on certain net investment income of certain U.S. holders that are individuals, estates or trusts. Such tax applies to the lesser of (i) the U.S. holder’s net investment income for the relevant taxable year and (ii) the excess of the U.S. holder’s adjusted gross income (with certain adjustments) over a specified threshold amount. Net investment income generally includes dividends and net gains from the disposition of shares of our Series B Preferred Stock or common stock. U.S. holders that are individuals, estates or trusts should consult their tax advisors regarding the effect, if any, of the Medicare tax on their ownership and disposition of our Series B Preferred Stock or common stock.
Information Reporting and Backup Withholding Tax
In general, information reporting will apply to payments of dividends on shares of our Series B Preferred Stock or common stock and proceeds of a disposition of shares of our Series B Preferred Stock or common stock to U.S. holders, other than certain exempt recipients such as corporations. Under U.S. federal income tax law, dividends and proceeds from the sale of shares of our Series B Preferred Stock or common stock paid to a U.S. holder (other than an exempt recipient) may be subject to “backup” withholding at the then applicable rate. Backup withholding generally applies to a U.S. holder if the holder (i) fails to furnish to us or our paying agent a correct social security number or other taxpayer identification number (TIN), or fails to furnish a certification of exempt status, (ii) has been notified by the IRS that the holder is subject to backup withholding as a result of the failure to properly report payments of interest or dividends or (iii) under certain circumstances, fails to provide a certified statement, signed under penalty of perjury, that the TIN provided is the holder’s correct number and that the holder is a U.S. person that is not subject to backup withholding. Backup withholding is not an additional tax. Any amounts withheld from payments to a U.S. holder under the backup withholding rules will be allowed as a credit against the holder’s U.S. federal income tax liability and may entitle the holder to a refund, provided that the required information is timely furnished to the IRS. Certain U.S. persons are exempt from backup withholding, including corporations, provided that their exemptions from backup withholding are properly established.
| 123 |
Non-U.S. Holders
The following is a summary of certain U.S. federal tax considerations applicable to a non-U.S. holder of our Series B Preferred Stock or warrants to purchase our common stock.
Distributions
Distributions treated as dividends for U.S. federal income tax purposes (as described above under “—U.S. Holders—Distributions), if any, that are paid to a non-U.S. holder with respect to shares of our capital stock will be subject to U.S. federal withholding tax at a 30% rate, or a lower rate prescribed by an applicable income tax treaty. To receive the benefit of a reduced treaty rate, a non-U.S. holder must provide the applicable withholding agent with an IRS Form W-8BEN, IRS Form W-8BEN-E, or other appropriate version of IRS Form W-8 certifying qualification for the reduced rate. Special certification and other requirements apply to certain non-U.S. holders that are pass-through entities rather than corporations or individuals. Dividends that are effectively connected with a trade or business carried on by a non-U.S. holder within the United States, and, to the extent an applicable tax treaty provides, attributable to a permanent establishment maintained by the non-U.S. holder in the United States, will generally be subject to U.S. federal income tax on a net basis at the individual or corporate rates generally applicable to U.S. holders, but will not be subject to U.S. withholding tax if certain certification requirements are satisfied. You can generally meet the certification requirements by providing a properly executed IRS Form W-8ECI or appropriate substitute form to the applicable withholding agent. A non-U.S. holder that is a corporation may also be subject to a "branch profits tax" at a 30% rate (or such lower rate as may be specified by an applicable tax treaty) on its "effectively connected earnings and profits," subject to certain adjustments, which will include effectively connected dividends. A non-U.S. holder of our Series B Preferred Stock or common stock may obtain a refund of any excess amounts withheld under these rules if the non-U.S. holder is eligible for a reduced rate of United States withholding tax and an appropriate claim for refund is timely filed with the IRS.
If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a non-taxable return of capital, up to the non-U.S. holder’s adjusted tax basis in the holder’s shares of our capital stock. Any remaining excess will be treated as capital gain, subject to the tax treatment described below in “—Sale, exchange, redemption or certain other taxable dispositions of our securities.” If we are not able to determine whether a distribution will exceed current and accumulated earnings and profits at the time a distribution is made, we may withhold tax on the entire amount of the distribution at the same rate as we would withhold on a dividend. However, a non-U.S. holder may obtain a refund of any excess withholding by filing an appropriate claim for refund with the IRS.
Any distribution described in this section would also be subject to the discussion below in “Foreign Account Tax Compliance Act.”
Sale, Exchange, Redemption or Certain Other Taxable Dispositions of Our Securities
Subject to the discussions below regarding backup withholding and the Foreign Account Tax Compliance Act, a non-U.S. holder generally will not be subject to U.S. federal income tax or withholding tax on gain realized upon a sale, exchange or other taxable disposition of shares of our Series B Preferred Stock or Common Stock unless: (i) the gain is effectively connected with the conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, is attributable to a U.S. permanent establishment or a fixed base), of the non-U.S. holder; (ii) the non-U.S. holder is a non-resident alien individual who is present in the United States for 183 days or more in the taxable year of that disposition, and certain other conditions are met; or (iii) we are or have been a “U.S. real property holding corporation” (USRPHC), for U.S. federal income tax purposes at any time within the shorter of the five-year period preceding the disposition and the non-U.S. holder’s holding period for our Series B Preferred Stock or Common Stock (as applicable), or the relevant period.
| 124 |
If the first exception applies, the non-U.S. holder generally will be subject to U.S. federal income tax on a net basis with respect to such gain in the same manner as if the holder were a resident of the United States. In addition, if the non-U.S. holder is a corporation for U.S. federal income tax purposes, such gains may, under certain circumstances, also be subject to the branch profits tax at a rate of 30% (or at a lower rate prescribed by an applicable income tax treaty).
If the second exception applies, the non-U.S. holder generally will be subject U.S. federal income tax at a rate of 30% tax on the gain from a disposition of our Common Stock, which may be offset by capital losses allocable to U.S. sources during the taxable year of disposition (even though the non-U.S. holder is not considered a resident of the United States).
With respect to the third exception above, we believe we currently are not, and we do not anticipate becoming, a USRPHC for U.S. federal income tax purposes. Because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property interests relative to the fair market value of our other trade or business assets and our foreign real property interests, there can be no assurances that we will not become a USRPHC in the future. Generally, a corporation is a USRPHC only if the fair market value of its U.S. real property interests (as defined in the Code) equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Even if we are or become a USRPHC, a non-U.S. holder would not be subject to U.S. federal income tax on a sale, exchange or other taxable disposition of our common stock by reason of our status as a USRPHC so long as (i) our common stock continues to be regularly traded on an established securities market (within the meaning of Section 897(c)(3) of the Code) during the calendar year in which such disposition occurs and (ii) such non-U.S. holder does not own and is not deemed to own (directly, indirectly, or constructively) more than 5% of our common stock at any time during the relevant period. If we are a USRPHC and the requirements of (i) or (ii) are not met, gain on the disposition of shares of our Series B Preferred Stock or common stock generally will be taxed in the same manner as gain that is effectively connected with the conduct of a U.S. trade or business, except that the branch profits tax will not apply.
Purchase of Units
For U.S. federal income tax purposes, the purchase of a unit by non-U.S. holders will be treated as the purchase of two components: a component consisting of one share of our Series B Preferred Stock and a component consisting of one warrant to purchase one share of our common stock. The purchase price for each unit will be allocated between its two components in proportion to the relative fair market value of each at the time the unit is purchased by the holder. This allocation of the purchase price for each unit will establish a holder’s initial tax basis for U.S. federal income tax purposes in the shares and warrants that comprise each unit.
Exercise of Warrants
A non-U.S. holder generally will not recognize gain or loss on the exercise of a warrant and related receipt of our shares of our common stock (unless cash is received in lieu of the issuance of a fractional share of our common stock). A non-U.S. holder’s initial tax basis in the shares of our common stock received upon the exercise of a warrant will be equal to the sum of (a) the non-U.S. holder’s tax basis in such warrant plus (b) the exercise price paid by the non-U.S. holder on the exercise of such warrant. A non-U.S. holder’s holding period for the shares of our common stock received upon the exercise of a warrant will begin on the date that such warrant is exercised by the non-U.S. holder.
In certain limited circumstances, a non-U.S. holder may be permitted to undertake a cashless exercise of warrants into shares of our common stock. The U.S. federal income tax treatment of a cashless exercise of warrants into shares of common stock is unclear, and the tax consequences of a cashless exercise could differ from the consequences upon the exercise of a warrant described in the preceding paragraph. Non-U.S. holders should consult their own tax advisors regarding the U.S. federal income tax consequences of a cashless exercise of warrants.
| 125 |
Certain Adjustments to the Warrants
An adjustment to the number of shares of our common stock that will be issued upon the exercise of a warrant, or an adjustment to the exercise price of a warrants, may be treated as a constructive distribution to a non-U.S. holder of the warrants if, and to the extent that, such adjustment has the effect of increasing the non-U.S. holder’s proportionate interest in our “earnings and profits” or assets, depending on the circumstances of such adjustment (for example, if the adjustment is to compensate for a distribution of cash or other property to our shareholders). Adjustments to the exercise price of warrants made pursuant to a bona fide reasonable adjustment formula that has the effect of preventing dilution of the interest of the holders of the warrants generally should not be considered to result in a constructive distribution. Any such constructive distribution would be taxable regardless whether there is an actual distribution of cash or other property. See the more detailed discussion of the rules applicable to distributions made by us under the heading “Distributions” above.
Conversion of Series B Preferred Stock
A non-U.S. holder generally will not recognize gain or loss upon the conversion of a share of Series B Preferred Stock into common stock. A non-U.S. holder’s initial tax basis in the shares of our common stock received upon the conversion of a share of Series B Preferred Stock will be equal to the non-U.S. holder’s tax basis in the share of Series B Preferred Stock. A non-U.S. holder’s holding period for the shares of our common stock received upon the conversion of a share of Series B Preferred Stock will include the non-U.S. holder’s holding period in the share of Series B Preferred Stock.
Information Reporting and Backup Withholding Tax
We must report annually to the IRS and to each non-U.S. holder the gross amount of the distributions on our Series B Preferred Stock or common stock paid to the holder and the tax withheld, if any, with respect to the distributions, regardless whether withholding is required. This information also may be made available under a specific treaty or agreement with the tax authorities in the country in which the non-U.S. holder resides or is established. A non-U.S. holder will generally be subject to backup withholding at the then applicable rate for dividends paid to such holder unless such holder furnishes a valid IRS Form W-8BEN (or such other applicable form and documentation as required by the Code or the Treasury regulations) certifying under penalties of perjury that the holder is a non-U.S. holder (and the payor does not have actual knowledge or reason to know that the holder is a United States person as defined under the Code), or otherwise establishes an exemption. Dividends paid to non-U.S. holders subject to U.S. federal withholding tax, as described above in “Distributions,” generally will be exempt from U.S. backup withholding.
Information reporting and, depending on the circumstances, backup withholding will apply to the payment of the proceeds of a sale or other disposition of shares of our Series B Preferred Stock or common stock by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies that the holder is not a United States person (as defined under the Code) and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected outside the U.S. through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker.
Copies of the information returns may be made available to the tax authorities in the country in which the non-U.S. holder resides or is incorporated under the provisions of an applicable treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a credit against a non-U.S. holder’s U.S. federal income tax liability, if any, and may entitle such holder to a refund, provided that an appropriate claim is timely filed with the IRS.
| 126 |
Foreign Account Tax Compliance Act
Under the Foreign Account Tax Compliance Act (FATCA), a 30% withholding tax will apply to dividends on, or gross proceeds from the sale or other disposition of, shares of our Series B Preferred Stock or common stock paid to certain non-U.S. entities (including financial intermediaries) unless various information reporting and due diligence requirements, which are different from and in addition to the certification requirements described elsewhere in this discussion, have been satisfied (generally relating to ownership by U.S. persons of interests in or accounts with those entities). The withholding rules applicable to payments of dividends on our Series B Preferred Stock or common stock were phased in beginning January 1, 2014. The withholding rules will apply to payments of gross proceeds from dispositions of U.S. capital stock beginning January 1, 2017.
Holders of our Series B Preferred Stock or common stock should consult their tax advisors regarding the possible impact of FATCA on their investment in our Series B Preferred Stock or common stock, including, without limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of the 30% withholding tax under FATCA.
| 127 |
Underwriting
We have entered into an underwriting agreement dated , 2016, with Ladenburg Thalmann & Co. Inc., as the underwriter (the “underwriter) named below and the sole book-running manager of this offering. Subject to the terms and conditions of the underwriting agreement, the underwriter has agreed to purchase the number of our securities set forth opposite its name below.
| Underwriter | Units |
| Ladenburg Thalmann & Co. Inc. | |
| Total: |
A copy of the underwriting agreement has been filed as an exhibit to the registration statement of which this prospectus is part.
We have been advised by the underwriter that it proposes to offer the units directly to the public at the public offering price set forth on the cover page of this prospectus. Any units sold by the underwriter to securities dealers will be sold at the public offering price less a selling concession not in excess of per unit. The underwriter may allow, and these selected dealers may re-allow, a concession of not more than per unit to other brokers and dealers.
The underwriting agreement provides that the underwriter’s obligation to purchase the units we are offering is subject to conditions contained in the underwriting agreement.
No action has been taken by us or the underwriter that would permit a public offering of the units to in any jurisdiction where action for that purpose is required. None of our securities included in this offering may be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sales of any of the securities offering hereby be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who receive this prospectus are advised to inform themselves about and to observe any restrictions relating to this offering of securities and the distribution of this prospectus. This prospectus is neither an offer to sell nor a solicitation of any offer to buy the units in any jurisdiction where that would not be permitted or legal.
The underwriter has advised us that they do not intend to confirm sales to any accounts over which they exercise discretionary authority.
Underwriting Discount and Expenses
The following table summarizes the underwriting discount and commission to be paid to the underwriter by us.
| Units | |||
| Public Offering Price | |||
| Underwriting Discount to be Paid to the Underwriter by Us | |||
| Proceeds to Us (Before Expenses) |
We estimate expenses payable by us in connection with this offering, exclusive of the underwriting discounts and commissions referred to above, will be approximately $ . We have also agreed to reimburse the underwriter for expenses up to $110,000 including an amount not to exceed $20,000 in connection with the clearance of this offering with the Financial Industry Regulatory Authority.
The units we are offering are being offered by the underwriter subject to certain conditions specified in the underwriting agreement.
One of our existing institutional investors, an investor affiliated with some of our directors, has a contractual obligation to purchase an aggregate of up to approximately $6,000,000 in units in this offering at the initial public offering price and on the same terms as the other purchasers in this offering.
| 128 |
Determination of Offering Price
Our common stock is currently traded on the OTCQB Marketplace under the symbol “XBIO.” On October 20, 2016, the closing price of our common stock was $4.40 per share. We have applied for the listing of our common stock on the NASDAQ Capital Market under the ticker symbol XBIO and will use our best efforts to have that listing effective on or before the closing. The warrants are not and will not be listed for trading on the NASDAQ Capital Market, or any other securities exchange. There is no established public trading market for our common stock and the share prices on an over-the-counter marketplace may not be indicative of the market price of our common stock on the NASDAQ Capital Market.
The public offering price of the securities offered by this prospectus will be determined by negotiation between us and the underwriter. Among the factors considered in determining the public offering price of the shares were:
| · | our history and our prospects; | |
| · | the industry in which we operate; | |
| · | our past and present operating results | |
| · | the previous experience of our executive officers; and | |
| · | the general condition of the securities markets at the time of this offering. |
The offering price stated on the cover page of this prospectus should not be considered an indication of the actual value of the units. That price is subject to change as a result of market conditions and other factors, and we cannot assure you that the units can be resold at or above the public offering price.
Lock-up Agreements
Our officers and directors have agreed with the underwriter to be subject to a lock-up period of 90 days following the date of this prospectus. This means that, during the applicable lock-up period, such persons may not offer for sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly, any shares of our common stock or any securities convertible into, or exercisable or exchangeable for, shares of our common stock. Certain limited transfers are permitted during the lock-up period if the transferee agrees to these lock-up restrictions. We have also agreed, in the underwriting agreement, to similar lock-up restrictions on the issuance and sale of our securities for 90 days following the closing of this offering, although we will be permitted to issue stock options or stock awards to directors, officers and employees under our existing plans. The lock-up period is subject to an additional extension to accommodate for our reports of financial results or material news releases. The underwriter may, in its sole discretion and without notice, waive the terms of any of these lock-up agreements.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Empire Stock Transfer, Inc.
Stabilization
In connection with this offering, the underwriter may engage in stabilizing transactions and syndicate covering transactions and purchases to cover positions created by short sales.
| · | Stabilizing transactions permit bids to purchase shares of common stock so long as the stabilizing bids do not exceed a specified maximum, and are engaged in for the purpose of preventing or retarding a decline in the market price of the common stock while the offering is in progress. | |
| · | Syndicate covering transactions involve purchases of common stock in the open market after the distribution has been completed in order to cover syndicate short positions. Since there is no over-allotment option, if the underwriter would have a naked short position, it can be closed out only by buying shares in the open market. A naked short position is more likely to be created if the underwriter is concerned that after pricing there could be downward pressure on the price of the shares in the open market that could adversely affect investors who purchase in the offering. |
| 129 |
Penalty bids permit the underwriter to reclaim a selling concession from a syndicate member when the security originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriter makes any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected on the NASDAQ Capital Market, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Indemnification
We have agreed to indemnify the underwriter and selected dealers against certain liabilities, including certain liabilities arising under the Securities Act, or to contribute to payments that the underwriter or selected dealers may be required to make for these liabilities.
| 130 |
Legal Matters
The validity of the issuance of our common stock offered in this prospectus will be passed upon for us by Westward Law Group, Las Vegas, Nevada. Goodwin Procter LLP, New York, New York has acted as counsel for the underwriter in connection with certain legal matters related to this offering.
Experts
The consolidated financial statements of Xenetic Biosciences, Inc. as of and for the year ended December 31, 2015 included in this prospectus have been so included in reliance on the report of Marcum LLP, an independent registered public accounting firm, given on the authority of said firm as experts in accounting and auditing. The financial statements as of and for the year ended December 31, 2014 audited by Ernst & Young LLP have been included in reliance on their report as experts in accounting and auditing.
The consolidated financial statements of Xenetic Biosciences, Inc. at December 31, 2014, and for the year then ended, appearing in this Prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, and at December 31, 2015, and for the year then ended December 31, 2015, by Marcum LLP, independent registered public accounting firm, as set forth in their respective reports thereon (which contains an explanatory paragraph describing conditions that raise substantial doubt about the Company's ability to continue as a going concern as described in Note 1 to the consolidated financial statements) appearing elsewhere herein, and are included in reliance upon such reports given on the authority of such firms as experts in accounting and auditing.
Changes in Registrant’s Certifying Accountant
On June 25, 2015, Ernst and Young LLP (Former Accountant) was dismissed as the Company’s independent registered public accounting firm. The Company approved the appointment of Marcum LLP (Marcum, or New Accountant) as its independent registered public accountant. The resolution to change accountants was approved by unanimous written consent of the Company’s board of directors.
The Former Accountant’s audit reports on the financial statements of the Company for the fiscal years ended December 31, 2014 and 2013 contained no adverse opinion or disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope or accounting principles, except that the audit reports on the financial statements of the Company for the fiscal year ended December 31, 2014 contained an uncertainty about the Company’s ability to continue as a going concern.
During the fiscal years ended December 31, 2014 and 2013, and through the interim period ended May 31, 2015, there were no “disagreements” (as such term is defined in Item 304 of Regulation S-K) with the Former Accountant on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedures, which disagreements if not resolved to the satisfaction of the Former Accountant would have caused them to make reference thereto in their reports on the financial statements for such periods.
During the fiscal years ended December 31, 2014 and 2013, and through the interim period ended May 31, 2015, there were the following “reportable events” (as such term is defined in Item 304 of Regulation S-K). As disclosed in Part I, Item 4 of the Company’s Form 10-Q for the quarterly period ended March 31, 2015, the Company’s management reported no material weaknesses regarding the Company’s internal controls over financial reporting.
Other than as disclosed above, there were no reportable events during the fiscal years ended December 31, 2014 and 2013, and through the interim period ended May 31, 2015. The Company’s Board of Directors discussed the subject matter of each reportable event with the Former Accountant. The Company authorized the Former Accountant to respond fully and without limitation to all requests of the New Accountant concerning all matters related to the audited period by the Former Accountant, including with respect to the subject matter of each reportable event.
Prior to retaining the New Accountant, the Company did not consult with the New Accountant regarding either: (i) the application of accounting principles to a specified transaction, either contemplated or proposed, or the type of audit opinion that might be rendered on the Company’s financial statements; or (ii) any matter that was the subject of a “disagreement” or a “reportable event” (as those terms are defined in Item 304 of Regulation S-K).
On June 25, 2015, the Company provided the Former Accountant with its disclosures in the Current Report on Form 8-K disclosing the dismissal of the Former Accountant and requested in writing that the Former Accountant furnish the Company with a letter addressed to the Commission stating whether or not they agree with such disclosures. The Former Accountant’s response was filed as an exhibit to the Current Report on Form 8-K, filed July 1, 2015.
| 131 |
Where You Can Find More Information
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the common stock we are offering by this prospectus. This prospectus is part of the registration statement and does not contain all of the information included in the registration statement and all of its exhibits, certificates and schedules. For further information pertaining to us and our common stock, you should refer to the registration statement and to its exhibits. Whenever we make reference in this prospectus to any of our contracts, agreements or other documents, the references are not necessarily complete, and you should refer to the exhibits attached to the registration statement for copies of the actual contract, agreement or other document.
We are subject to the informational and reporting requirements of the Securities Exchange Act of 1934, as amended, and have filed and will file annual, quarterly and current reports, proxy statements and other information with the SEC. You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website (http://www.sec.gov). You may also read and copy any document we file with the SEC at its public reference facility at 100 F Street, N.E., Room 1580, Washington, D.C. 20549.
You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
INCORPORATION OF DOCUMENTS BY REFERENCE
We have elected to “incorporate by reference” certain information into this prospectus. By incorporating by reference, we are disclosing important information to you by referring you to documents we have filed separately with the Securities and Exchange Commission, or “SEC.” The information incorporated by reference is deemed to be part of this prospectus, except for information incorporated by reference that is superseded by information contained in this prospectus. Any statement contained in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained herein modifies or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus. The following documents filed with the SEC are incorporated by reference in this prospectus (Commission File No. 333-211249), except for any document or portion thereof deemed to be “furnished” and not filed in accordance with SEC rules:
| ● | Quarterly Report on Form 10-Q for the quarter ended March 31, 2016 filed on May 16, 2016 and the quarter ended June 30, 2016 filed on August 15, 2016; and |
| ● | Current Report on Form 8-K filed on February 20, 2016, June 1, 2016, July 8, 2016, July 12, 2016, and September 23, 2016. |
In addition, all documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, prior to the termination of the offering shall be deemed to be incorporated by reference into this prospectus. You should rely only on the information contained in this prospectus, as updated and supplemented by any prospectus supplement, or that information to which this prospectus or any prospectus supplement has referred you by reference. We have not authorized anyone to provide you with any additional information.
You may request and obtain a copy of any of the filings incorporated herein by reference, at no cost, by writing or telephoning us at the following address or phone number:
Xenetic Biosciences, Inc.
99 Hayden Avenue - Suite 230
Lexington, MA 02421
Attention: Chief Executive Officer
| 132 |
Index to Financial Statements
| Condensed Consolidated Financial Statements: | |
| Condensed Consolidated Balance Sheets as of June 30, 2016 (Unaudited) and December 31, 2015 | F-2 |
| Condensed Consolidated Statements of Comprehensive Loss (Unaudited) for the three months and six months ended June 30, 2016 and 2015 | F-2 |
| Condensed Consolidated Statements of Cash Flows (Unaudited) for the six months ended June 30, 2016 and 2015 | F-3 |
| Notes to Condensed Consolidated Financial Statements | F-4 |
| F-1 |
XENETIC BIOSCIENCES, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
| JUNE 30, 2016 | DECEMBER 31, 2015 | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 240,786 | $ | 132,229 | ||||
| Restricted cash | 66,510 | 66,510 | ||||||
| Prepayment on acquisition | – | 3,744,517 | ||||||
| Prepaid expenses and other | 171,199 | 247,298 | ||||||
| Total current assets | 478,495 | 4,190,554 | ||||||
| Property and equipment, net | 58,471 | 62,021 | ||||||
| Goodwill | 3,283,379 | 3,283,379 | ||||||
| Indefinite-lived intangible assets | 9,243,128 | 9,243,128 | ||||||
| Other assets | 97,238 | 129,306 | ||||||
| Total assets | $ | 13,160,711 | $ | 16,908,388 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 2,021,205 | $ | 1,788,521 | ||||
| Accrued expenses | 1,607,125 | 1,487,046 | ||||||
| Hybrid debt instrument, net | – | 3,652,749 | ||||||
| Other current liabilities | 19,647 | 19,098 | ||||||
| Loans due to related parties | 152,529 | 395,000 | ||||||
| Total current liabilities | 3,800,506 | 7,342,414 | ||||||
| Deferred tax liability | 2,918,518 | 2,918,518 | ||||||
| Other liabilities | 29,446 | 38,791 | ||||||
| Total liabilities | 6,748,470 | 10,299,723 | ||||||
| Stockholders' equity: | ||||||||
| Common stock, $0.001 par value; 45,454,546 shares authorized as of June 30, 2016 and December 31, 2015; 9,348,757 and 4,909,685 shares issued as of June 30, 2016 and December 31, 2015, respectively; 9,024,872 and 4,585,800 shares outstanding as of June 30, 2016 and December 31, 2015, respectively | 9,348 | 4,909 | ||||||
| Additional paid in capital | 150,905,035 | 99,763,101 | ||||||
| Accumulated deficit | (139,474,696 | ) | (88,131,899 | ) | ||||
| Accumulated other comprehensive income | 253,734 | 253,734 | ||||||
| Treasury stock | (5,281,180 | ) | (5,281,180 | ) | ||||
| Total stockholders' equity | 6,412,241 | 6,608,665 | ||||||
| Total liabilities and stockholders' equity | $ | 13,160,711 | $ | 16,908,388 | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
| F-2 |
XENETIC BIOSCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(Unaudited)
| THREE MONTHS ENDED JUNE 30, | SIX MONTHS ENDED JUNE 30, | |||||||||||||||
| 2016 | 2015 | 2016 | 2015 | |||||||||||||
| Operating costs and expenses: | ||||||||||||||||
| Research and development | $ | (2,205,213 | ) | $ | (555,740 | ) | $ | (2,634,494 | ) | $ | (1,590,823 | ) | ||||
| IPR&D expense | (39,500,000 | ) | – | (39,500,000 | ) | – | ||||||||||
| General and administrative | (1,557,677 | ) | (803,399 | ) | (2,980,043 | ) | (1,738,625 | ) | ||||||||
| Loss from operations | (43,262,890 | ) | (1,359,139 | ) | (45,114,537 | ) | (3,329,448 | ) | ||||||||
| Other income (expense): | ||||||||||||||||
| Change in fair value of derivative liability | 1,769,275 | – | 1,905,289 | – | ||||||||||||
| Loss on issuance of hybrid debt instrument | – | – | (1,584,218 | ) | – | |||||||||||
| Loss on conversion of debt | (6,187,337 | ) | – | (6,187,337 | ) | – | ||||||||||
| Other income (expense) | 12,863 | 234,453 | (13,551 | ) | (225,515 | ) | ||||||||||
| Interest income | 13 | 914 | 27 | 1,088 | ||||||||||||
| Interest expense | (103,086 | ) | (1,386 | ) | (348,470 | ) | (2,512 | ) | ||||||||
| (4,508,272 | ) | 233,981 | (6,228,260 | ) | (226,939 | ) | ||||||||||
| Net loss | (47,771,162 | ) | (1,125,158 | ) | (51,342,797 | ) | (3,556,387 | ) | ||||||||
| Other comprehensive loss from foreign currency translation adjustment | – | (338,875 | ) | – | (327,054 | ) | ||||||||||
| Total comprehensive loss | $ | (47,771,162 | ) | $ | (1,464,033 | ) | $ | (51,342,797 | ) | $ | (3,883,441 | ) | ||||
| Net loss per share of common stock, basic and diluted | $ | (6.12 | ) | $ | (0.27 | ) | $ | (8.28 | ) | $ | (0.84 | ) | ||||
| Weighted-average shares of common stock outstanding, basic and diluted | 7,804,187 | 4,221,328 | 6,197,776 | 4,221,328 | ||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
| F-3 |
XENETIC BIOSCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
| SIX MONTHS ENDED JUNE 30, | ||||||||
| 2016 | 2015 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net Loss | $ | (51,342,797 | ) | $ | (3,556,387 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| IPR&D expense | 39,500,000 | |||||||
| Depreciation and amortization | 18,164 | 38,828 | ||||||
| Amortization of hybrid debt instrument discount | 204,003 | – | ||||||
| Non-cash interest expense | 142,929 | – | ||||||
| Share-based payments | 899,621 | 144,495 | ||||||
| Warrant expense for services | 1,118,642 | 92,703 | ||||||
| Change in fair value of derivative liability | (1,905,289 | ) | – | |||||
| Loss on issuance of hybrid debt instrument | 1,584,218 | – | ||||||
| Loss on conversion of debt | 6,187,337 | – | ||||||
| Foreign currency translation | – | 344,676 | ||||||
| Other non-cash transactions | – | (129,328 | ) | |||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other assets | 36,990 | 148,580 | ||||||
| Accounts payable, accrued expenses and other liabilities | 421,823 | 686,597 | ||||||
| Net cash used in operating activities | (3,134,359 | ) | (2,229,836 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchases of property and equipment | (14,613 | ) | (1,663 | ) | ||||
| Disposition of property and equipment | – | 6,245 | ||||||
| Net cash (used in) provided by investing activities | (14,613 | ) | 4,583 | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from issuance of promissory note | 3,500,000 | 100,000 | ||||||
| Payments on loan from related party | (242,471 | ) | – | |||||
| Net cash provided by financing activities | 3,257,529 | 100,000 | ||||||
| Effect of exchange rate change on cash | – | (80,668 | ) | |||||
| Net change in cash, excluding restricted cash | 108,557 | (2,205,921 | ) | |||||
| Cash at beginning of period | 132,229 | 2,507,401 | ||||||
| Cash at end of period | $ | 240,786 | $ | 301,480 | ||||
| SUPPLEMENTAL SCHEDULE OF NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Convertible debt paid in common stock | $ | 6,500,000 | – | |||||
| Interest paid in common stock | $ | 227,829 | $ | – | ||||
| Non-cash issuance of warrants in connection with debt | $ | 1,701,214 | $ | – | ||||
| Non-cash recording of derivative liability in connection with debt | $ | 3,346,423 | $ | – | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
| F-4 |
XENETIC BIOSCIENCES, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
| 1. | The Company |
Background
Xenetic Biosciences, Inc. (the “Company”), incorporated in the state of Nevada and based in Lexington, Massachusetts, is a clinical stage biopharmaceutical company that is focused on the discovery, development and planned commercialization of a new generation of human drug therapies for the treatment of a variety of conditions including anemia, endometrial cancer, refractory acute myeloid leukemia, Cystic Fibrosis and certain other cancers based upon its proprietary and patented drug delivery platform systems and drug development collaborations with major third party pharmaceutical companies around the world.
We incorporate our patented and proprietary technologies into a number of drug candidates currently under development either in-house or with biotechnology and pharmaceutical collaborators in order to create what we believe will be the next-generation biologic drugs and therapeutics. While we primarily focus on researching and developing orphan oncology drugs, we also have significant interests in drugs being developed by our collaborators to treat, among others, hemophilia and anemia. Our four core proprietary technologies are:
| PolyXen™ | An enabling biological platform technology designed to extend the circulation in the human body for a variety of existing drug molecules and, thereby, to create potentially superior next generation drug candidates. PolyXen is based on the concept of polysialylation and utilizes polysialic acid, or PSA, which is a biopolymer, comprising a chain of sialic acids molecules. PSA is a natural constituent of the human body, though we obtain our PSA from a bacterial source.
|
| Virexxa® | A small molecule therapeutic with the potential to confer sensitivity to cancer cells to hormone therapeutics that are otherwise insensitive to such treatments. Virexxa, sodium cridanimod, belongs to a class of low-molecular weight synthetic interferon inducers. In addition to its immunomodulatory properties, Virexxa has been shown to increase levels of progesterone receptor expression in tumor tissue of patients who are progesterone receptor deficient, and thus may restore sensitivity of non-responsive endometrial cancers to hormonal (e.g., progestin) therapy. Based on preclinical observations, Virexxa may also be therapeutically relevant in other hormone-resistant cancers, such as triple-negative breast cancer. Virexxa has been granted an Orphan Drug Designation by the FDA, for treatment of progesterone receptor negative endometrial cancer in conjunction with progesterone therapy.
|
| OncoHist™ | A novel therapeutic platform technology that utilizes the properties of modified human histone H1.3 for targeted cell necrosis or apoptosis programed cell death, which may enable OncoHist to treat a broad range of cancer indications. OncoHist, unlike many competing oncology therapies, is based on a molecule occurring naturally in the human body, in the cell nucleus, and is therefore expected to be less toxic and immunogenic than other oncology therapies.
|
| ImuXen™ | A novel liposomal co-entrapment encapsulation technology designed to maximize both cell and immune system mediated responses. The technology is based on the co-entrapment of the nominated antigen(s) in a liposomal vesicle. The technology when applied may create new vaccines and improve the use and efficacy of certain existing human vaccines. |
These proprietary technologies may address unmet needs, improve the performance of existing drugs, and create new patentable drug candidates. All of our drug candidates are in the development stage and none has yet received regulatory approval for marketing in the U.S. by the FDA or by any applicable agencies in other countries.
Going Concern and Management’s Plan
While these condensed consolidated financial statements have been prepared on a going concern basis, if the Company does not successfully raise additional working capital, there can be no assurance that the Company will be able to continue its operations and these conditions raise substantial doubt about its ability to continue as a going concern. The accompanying condensed consolidated financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
| F-5 |
In March 2016, the Company engaged an investment banking firm to assist with a proposed sale of the Company’s securities. Though a positive outcome cannot be assured, as of this filing the Company is in late-stage negotiations to effect a public offering as described herein. The Company is optimistic that it will be successful in obtaining financing; however there can be no assurance that it will be able to do so or, if it is able to, that is can do so under commercially reasonable terms. In the event the Company is unsuccessful in this proposed sale, the Company will plan to rely upon proceeds from the sale of up to an additional $6.0 million in securities to PJSC Pharmsynthez (“Pharmsynthez”) as provided for in the November 2015 Asset Purchase Agreement with Pharmsynthez and AS Kevelt (“Kevelt”).
| 2. | Summary of Significant Accounting Policies |
Preparation of Interim Financial Statements
The accompanying condensed consolidated financial statements were prepared by the Company pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) and, in the opinion of management, include all normal and recurring adjustments necessary to present fairly the results of the interim periods shown. Certain information and footnote disclosures normally included in financial statements prepared in accordance with US GAAP have been condensed or omitted pursuant to such SEC rules and regulations. Management believes that the disclosures made are adequate to make the information presented not misleading. The results for the interim periods are not necessarily indicative of results for the full year. The condensed consolidated financial statements contained herein should be read in conjunction with the consolidated financial statements and notes thereto included in the Company’s 2015 Annual Report on Form 10-K.
Certain prior period amounts have been reclassified to conform to the presentation of the current period.
Principles of Consolidation
The financial statements of the Company include the accounts of Xenetic Biosciences (UK) Limited (“Xenetic UK”) and its wholly owned subsidiaries: Lipoxen Technologies Limited, Xenetic Bioscience, Incorporated, and SymbioTec GmbH (“SymbioTec”). All material intercompany balances and transactions have been eliminated on consolidation.
| 3. | Significant Strategic Drug Development Collaborations – Related Parties |
The Company has entered into various research, development, license and supply agreements with Shire plc (“Shire”), formerly Baxalta Incorporated (a spinoff of the biopharmaceuticals business from Baxter Healthcare SA and Baxter Healthcare Corporation), SynBio LLC (“SynBio”), Serum Institute of India (“Serum”) and Pharmsynthez. The Company and its collaborative partners continue to engage in research and development activities with no resultant commercial products through June 30, 2016. No amounts were recognized as revenue related to these agreements during the six months ended June, 2016 or 2015.
| 4. | Property and Equipment, net |
Property and equipment, net consists of the following:
| June 30, 2016 | December 31, 2015 | |||||||
| Laboratory equipment | $ | 264,583 | $ | 249,969 | ||||
| Office and computer equipment | 35,190 | 35,190 | ||||||
| Leasehold improvements | 26,841 | 26,841 | ||||||
| Furniture and fixtures | 20,263 | 20,263 | ||||||
| Property and equipment – at cost | 346,877 | 332,263 | ||||||
| Less accumulated depreciation | (288,406 | ) | (270,242 | ) | ||||
| Property and equipment – net | $ | 58,471 | $ | 62,021 | ||||
Depreciation expense was $9,082 and $8,551 for the three months ended June 30, 2016 and 2015, respectively, and $18,164 and $38,828 for the six months ended June 30, 2016 and 2015, respectively.
| F-6 |
| 5. | Hybrid Debt Instrument |
On July 1, 2015, the Company entered into a Securities Purchase Agreement (the “SPA”) with Pharmsynthez providing for the issuance of a minimum of a $3 million 10% Senior Secured Collateralized Convertible Promissory Note (the “SPA Note”). The SPA also provides for the issuance of certain warrants up to the amount of the SPA Note. The convertible debt and its embedded debt-like features were recorded on the face of the condensed consolidated balance sheet within current liabilities as an aggregate hybrid debt instrument.
On November 13, 2015, the Company entered into an Asset Purchase Agreement (the “APA”) with Pharmsynthez providing for the issuance of a minimum of a $3.5 million 10% Senior Secured Collateralized Convertible Promissory Note (the “APA Note”) and the transfer to the Company of certain intellectual property rights with respect to Virexxa in exchange for, among others, 111.5 million shares of our common stock. The APA also provides for the issuance of certain warrants covering up to half the amount of the APA Note. During the quarter ended March 31, 2016, the Company issued $3.5 million of convertible debt as well as the associated warrants, both in connection with the APA Note. The convertible debt and its embedded debt-like features were recorded on the face of the condensed consolidated balance sheet within current liabilities as an aggregate hybrid debt instrument. See also Note 12. Subsequent Events.
On April 22, 2016, Pharmsynthez converted all convertible notes (in the principal amount of $6.5 million plus accrued interest of approximately $228,000), issued by the Company to Pharmsynthez in 2015 and 2016. The conversion rate was $4.95 per share. As such, the Company issued to Pharmsynthez 1,373,036 shares of common stock in connection with conversion of the convertible notes. The related embedded derivatives, which had been bifurcated from the host debt and accounted for separately, were settled by action of the conversion. The Company recognized a net loss on conversion, including a final mark-to-market of the compound derivative, of $4.4 million, which is recorded in other expense in the condensed consolidated statement of comprehensive loss for the three and six months ended June 30, 2016.
Interest expense related to the SPA Note and the APA Note of approximately $102,000 and $347,000 was recognized in the condensed consolidated statement of comprehensive loss for the three and six months ended June 30, 2016, respectively.
| 6. | Fair Value Measurements |
ASC Topic 820, Fair Value Measurement, defines fair value as the price that would be received to sell an asset or be paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Company applies the following fair value hierarchy, which prioritizes the inputs used to measure fair value into three levels and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement. Level 1 inputs are quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. Level 2 utilizes quoted market prices in markets that are not active, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency. Level 3 inputs are unobservable inputs for the asset or liability in which there is little, if any, market activity for the asset or liability at the measurement date.
The Company’s cash and restricted cash are measured at fair value on a recurring basis and are classified as Level 1 in the fair value hierarchy. The carrying amount of certain of the Company’s financial instruments approximate fair value due to their short maturities. The Company’s derivative liabilities are measured at fair value on a recurring basis and are classified as Level 3 in the fair value hierarchy.
The following table provides a summary of the changes in fair value of the compound derivative measured at fair value on a recurring basis using significant unobservable inputs during the six months ended June 30, 2016.
| Balance as of January 1, 2016 | $ | 3,544,222 | ||
| Issuances of compound derivative instrument | 3,346,423 | |||
| Change in fair value of compound derivative instrument | (1,905,289 | ) | ||
| Settlement of derivative instrument through conversion of debt host | (4,985,356 | ) | ||
| Balance as of June 30, 2016 | $ | – |
There were no financial instruments classified as Level 3 in the fair value hierarchy during the six months ended June 30, 2015.
| F-7 |
| 7. | Stockholders’ Equity |
Reverse Stock Split
On May 16, 2016, our board of directors approved a reduction, on a 1 for 33 basis, in our authorized common stock, par value $0.001, along with a corresponding and proportional decrease in the number of shares issued and outstanding. This reduction was filed with the Nevada Secretary of State on May 18, 2016, but required a review by FINRA before becoming effective in the market. On May 31, 2016, FINRA announced that this change took effect in the over-the-counter securities markets on June 1, 2016.
All share information provided herein reflects the effect of the reverse stock split.
Common Stock
In November 2015, the Company agreed to issue 3.38 million shares of common stock in connection with the APA. In December 2015, 0.33 million shares of common stock were issued to Dr. Genkin and Mr. Surkhov pursuant to the APA.
On April 29, 2016, the Company closed on the APA with an effective date of April 27, 2016, acquiring in-process research and development (“IPR&D”) related to certain intellectual property rights with respect to the immunomodulator product Virexxa held by Kevelt including the grant of the worldwide right to develop, market and license Virexxa for certain uses. In connection with the closing of the APA, the Company issued 3.05 million shares of its common stock to Pharmsynthez and, because there was no alternative use for the IPR&D, the Company recognized $39.5 million of expense based on the fair value of Virexxa IP received, which was determined to be more reliably measured than the related equity consideration. Included in the $39.5 million expense was the $3.74 million prepayment recorded in 2015.
Financing Warrants
In connection with the Company’s issuance of the APA Note in March 2016, the Company issued a warrant to purchase 0.35 million shares of common stock in accordance with the terms of the APA (the “Warrant”). The Warrant has a five-year term and is exercisable commencing March 31, 2016. The exercise price per share under the Warrant is the lessor of $6.60 or 120% of the Public Offering price, in the event there is a Public Offering, as defined in the APA. If the APA Note is not repaid or converted on or before six months from the date of issuance, the Holder will be issued an additional warrant to purchase 0.35 million shares of common stock under the same terms as the Warrant. The Company determined there is a low probability that the Note will not be repaid or converted within the period six months from the date of issuance and, therefore, did not account for the additional warrant as issued. The fair value of the warrant was calculated using the Black-Scholes option pricing model. The key valuation assumptions used consist of the Company’s stock price, a risk free rate of 1.42% and an expected volatility of 135%. Using an allocation of the APA Note proceeds between the relative fair values of the Warrants and the APA Note, the Company recorded the Warrants at a value of $1.7 million on the condensed consolidated balance sheet as equity paid-in-capital.
| 8. | Share-Based Compensation |
Total share-based compensation related to stock options, common stock awards, and non-financing warrants was $1,731,803 and $89,970 for the three months ended June 30, 2016 and 2015, respectively, and $2,018,263 and $237,198 for the six months ended June 30, 2016 and 2015, respectively.
Share-based compensation expense is classified in the condensed consolidated statements of comprehensive loss as follows:
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| 2016 | 2015 | 2016 | 2015 | |||||||||||||
| Research and development expenses | $ | 1,427,691 | $ | 51,736 | $ | 1,234,240 | $ | 150,881 | ||||||||
| Administrative expenses | 304,112 | 38,234 | 784,023 | 86,317 | ||||||||||||
| $ | 1,731,803 | $ | 89,970 | $ | 2,018,263 | $ | 237,198 | |||||||||
| F-8 |
Employee Stock Options
During the six months ended June 30, 2016 and 2015, the Company granted 12,122 employee stock options. The key valuation assumptions used consisted of the Company’s stock price, a risk free rate of 0.54% and an expected volatility of 123%. There were no employee stock options granted during the same period in 2015. During the six months ended June 30, 2016, the Company extended the exercise expiration date of certain former employee stock option awards resulting in a change in incremental value of approximately $24,000 which was charged to administrative expense. The Company recognized compensation expense related to employee stock options of $310,469 and $20,668 during the three months ended June 30, 2016 and 2015, respectively, and $765,026 and $84,303 during the six months ended June 30, 2016 and 2015, respectively.
Non-Employee Stock Options
No non-employee stock options were granted during the six months ended June 30, 2016 or 2015 and no non-employee stock options were exercised during the six months ended June 30, 2016 or 2015. The Company recognized compensation expense related to non-employee stock options of $3,474 and $4,755 during the three months ended June 30, 2016 and 2015, respectively, and $2,503 and $9,193 during the six months ended June 30, 2016 and 2015, respectively.
Common stock awards
The Company granted 9,581 and 925 common stock awards during the three months ended June 30, 2016 and 2015, respectively, and 11,939 and 2,043 common stock awards during the six months ended June 30, 2016 and 2015, respectively, based on the value of the services provided and the average stock price during each respective period. The Company recognized compensation expense related to common stock awards of $50,000 and $25,500 during the three months ended June 30, 2016 and 2015, respectively, and $107,790 and $51,000 during the six months ended June 30, 2016 and 2015, respectively.
Warrants
In connection with certain of the Company’s collaboration agreements and consulting arrangements, the Company has issued warrants to purchase shares of common stock. On May 16, 2016, the Company modified the exercise price of 150,307 performance-based warrants held by Serum and individuals related to Serum from $25.41 to $7.92 and resulted in an incremental value expense of $204,000. Additionally, the Company issued 212,122 warrants to purchase shares of common stock to Serum with an exercise price of $7.92. The new warrants were fully vested and the Company recognized $1.37 million of expense related to the grant.
As of June 30, 2016, and December 31, 2015, warrants to purchase 758,347 shares of common stock were outstanding. These warrants were fair valued at each issuance date using the Black-Scholes option pricing model. Warrants for which a measurement has not been reached are subject to re-measurement at each reporting period until the measurement date is reached. Expense is recognized on a straight-line basis over the expected service period or at the date of issuance, if there is not a service period. Expense for the six months ended June 30, 2016, was $1.1 million including the incremental value recognized for the warrant modification. The Company issued no warrants in connection with collaboration agreements and consulting services during the three and six months ended June 30, 2015.
| 9. | Income Taxes |
During the six months ended June 30, 2016 and 2015, there was no provision for income taxes as the Company incurred losses during both periods. Deferred tax assets and liabilities reflect the net tax effect of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The Company records a valuation allowance against its deferred tax assets as the Company believes it is more likely than not the deferred tax assets will not be realized. The valuation allowance against deferred tax assets was approximately $16.5 million and $15.3 million as of June 30, 2016 and December 31, 2015, respectively.
As of June 30, 2016, and December 31, 2015, the net deferred tax liability of $2,918,518 on the condensed consolidated balance sheets is related to book and tax basis differences for intangible assets with indefinite lives that were acquired in the January 2012 acquisition of SymbioTec. In accordance with ASC 740-10-30-18, the deferred tax liability related to the intangible assets cannot be used to offset deferred tax assets when determining the amount of the valuation allowance for deferred tax assets which are not more-likely-than-not to be realized. This results in a net deferred tax liability, even though the Company has a full valuation allowance on its other net deferred tax assets. This net deferred tax liability will continue to be reflected on the balance sheet until the related intangible assets are no longer held by the Company.
As of June 30, 2016 and December 31, 2015, the Company did not record any unrecognized tax positions.
| F-9 |
| 10. | Commitments |
In August 2013, the Company entered into an agreement to lease office and laboratory space in Lexington, Massachusetts under an operating lease with a commencement date of January 1, 2014 and a termination date of January 31, 2019. With the execution of this lease, the Company is required to maintain a $66,000 letter of credit as a security deposit. In connection with the Lexington lease, the Company recorded $76,107 as prepaid rent as of June 30, 2016, with $46,646 recorded as a non-current asset. The Company also incurred a liability of $89,074 with respect to the Company’s contribution to the landlord’s leasehold improvements, of which $47,743 is outstanding as of June 30, 2016, with $29,446 recorded as a non-current liability, respectively. This liability is repayable as additional rent expense over the term of the lease and bears interest at 6%. The Company also leased office space in London, UK during 2014, however the lease was terminated in March 2015 in accordance with the terms of the lease.
| 11. | Related Party Transactions |
In May 2011, the Company received a short term unsecured loan facility of up to $1.7 million from SynBio, an affiliate of the Company, of which $152,529 and $395,000 was outstanding as of June 30, 2016 and December 31, 2015, respectively. In connection with the APA, the Company made a series of payments during the first two quarters of 2016 totaling $242,471 to creditors of Kevelt. Pursuant to the APA such payments are considered direct offsets to the loan with SynBio. No payments were made during the six months ended June 30, 2015. The loan had an interest rate of 8.04% per annum as of the date of grant, with interest payable upon repayment of the loan, which was to be seven months after the closing date of the loan. During 2012, the loan matured and it was agreed by both parties that the loan can be called due with full repayment of the outstanding principal including accrued interest upon future agreement by both parties. It was also agreed as of July 1, 2012 that no further interest on the outstanding loan balance would be accrued. The loan is recorded in “Loans due to related parties” within current liabilities.
The Company has entered into various research, development, license and supply agreements with Shire, SynBio, Serum and Pharmsynthez, each a related party whose relationship and ownership has not materially changed from that disclosed in our 10-K/A filed April 29, 2016.
| 12. | Subsequent Events |
On July 1, 2016, the Company received total proceeds of $0.5 million in connection with the APA financing arrangement. The APA provided for the issuance of certain warrants to purchase a number of share of the Company’s common stock equal to 50% of the number of shares issuable under the APA Notes. The Warrant has a five-year term and is exercisable upon grant. The exercise price per share under the Warrant is the lessor of $6.60 or 120% of the Capital Raise price, in the event there is a Capital Raise. If the APA Note is not repaid or converted on or before six months from the date of issuance, the Holder will be issued an additional warrant under the same terms as the Warrant.
Mr. M. Scott Maguire is our Chief Executive Officer. Mr. Maguire current annual salary is $505,735 pursuant to his written employment agreement with the Company. Of Mr. Maguire’s 2015 salary amount and 2016 salary amount through today, fifty percent (50%) has been paid in cash and fifty percent (50%) has been deferred and accrued pursuant to an unwritten arrangement between us and Mr. Maguire. On July 1, 2016, we issued a convertible promissory note in the amount of $369,958 and warrants to purchase 37,369 shares of our common stock at the Exercise Price to Mr. Maguire for the deferred salary. We also entered into a Deferred Salary Security Agreement with Mr. Maguire, pursuant to which Mr. Maguire agreed to continue to defer fifty percent (50%) of his salary until the earlier to occur of: (i) the closing of a public offering of our securities concurrent to a NASDAQ listing, or (ii) September 30, 2016 (the “Deferral End Date”). All deferred salary shall become due and payable on the Deferral End Date. As security for the payment of the deferred salary, we granted Mr. Maguire a continuing subordinated security interest in our assets, including all inventory, accounts, accounts receivable, equipment, trademarks, contracts, copyrights and general intangibles.
On August 26 and September 9, 2016 we issued convertible promissory notes in the amount of $178,000 and $322,000, respectively, to Pharmsynthez. The notes are convertible into shares of our common stock at any time at a conversion price of $4.00 per share (subject to price protection and usual and customary adjustments) or may be applied toward the Offering, at the option of Pharmsynthez.
On September 23, 2016, SynBio, one of our largest shareholders exchanged 970,000 shares of common stock in the Company for an equal number of shares of Series A Preferred Stock.
| F-10 |
Index to Financial Statements
|
Report of Independent Registered Public Accounting Firm (Marcum LLP) |
F-12 | |
| Report of Independent Registered Public Accounting Firm (Ernst & Young LLP) | F-13 | |
| Consolidated Balance Sheets as of December 31, 2015 and 2014 | F-14 | |
| Consolidated Statements of Comprehensive Loss for the years ended December 31, 2015 and 2014 | F-15 | |
| Consolidated Statements of Cash Flows for the years ended December 31, 2015 and 2014 | F-16 | |
| Consolidated Statements of Changes in Stockholders’ Equity for the years ended December 31, 2015 and 2014 | F-17 | |
| Notes to the Consolidated Financial Statements | F-18 |
| F-11 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Audit Committee of the
Board of Directors and Shareholders of
Xeentic Biosciences, Inc.
We have audited the accompanying consolidated balance sheet of Xenetic Biosciences, Inc. (the “Company”) as of December 31, 2015, and the related consolidated statements of comprehensive loss, changes in stockholders’ equity and cash flows for the year ended December 31, 2015. Our audit also includes the financial statement schedule. These consolidated financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Xenetic Biosciences, Inc., as of December 31, 2015 and the consolidated results of its operations and its cash flows for the year ended December 31, 2015 in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has had recurring net losses and continues to experience negative cash flows from operations. These conditions raise substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters also are described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
The financial statements of Xenetic Biosciences, Inc. as of and for the year ended December 31, 2014, were audited by other auditors whose report dated April 15, 2015, expressed an unmodified opinion on those financial statements. As discussed in Notes 11 and 2 to the financial statements, the Company has adjusted its 2014 financial statements to retrospectively apply the change in par value and the reverse stock split that occurred subsequent to the year ended December 31, 2015. The other auditors reported on the financial statements before the retrospective adjustments.
As part of our audit of the 2015 financial statements, we also audited the adjustments to the 2014 financial statements to retroactively apply the change in par value and the reverse stock split that occurred subsequent to the year ended December 31, 2015 as described in Notes 11 and 2. In our opinion, such adjustments are appropriate and have been properly applied. We were not engaged to audit, review, or apply any procedures to Xenetic Biosciences’ 2014 financial statements other than with respect to the adjustments and, accordingly, we do not express an opinion or any other form of assurance on the 2014 financial statements as whole.
/s/ Marcum LLP
Boston, MA
March 30, 2016, except for Note 2, as to which the date is June 23, 2016
| F-12 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Shareholders of Xenetic Biosciences, Inc.
We have audited, before the effects of the adjustments to retrospectively apply the change in par value and the reverse stock split as described in Notes 11 and 2, the consolidated balance sheet of Xenetic Biosciences, Inc. (the “Company”) as of December 31, 2014, and the related consolidated statements of comprehensive loss, changes in stockholders’ equity, and cash flows for the year then ended (the 2014 financial statements before the effects of the adjustments discussed in Notes 11 and 2 are not presented herein). Our audit also includes the financial statement schedule. The 2014 financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audit provide a reasonable basis for our opinion.
In our opinion, the 2014 financial statements, before the effects of the adjustments to retrospectively apply the change in par value and the reverse stock split described in Notes 11 and 2, present fairly, in all material respects, the consolidated financial position of Xenetic Biosciences, Inc. as of December 31, 2014, and the consolidated results of its operations and its cash flows for the year then ended in conformity with US generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As disclosed in Note 1 to the Financial Statements, the Company’s recurring losses from operations and its requirement to raise funds to continue operations beyond April 2015, raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The 2014 consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
We were not engaged to audit, review, or apply any procedures to the adjustments to retrospectively apply the change in par value and the reverse stock split described in Notes 11 and 2 and, accordingly, we do not express an opinion or any other form of assurance about whether such adjustments are appropriate and have been properly applied. Those adjustments were audited by Marcum LLP.
/s/ Ernst & Young LLP
Reading, United Kingdom
April 15, 2015
| F-13 |
XENETIC BIOSCIENCES, INC.
CONSOLIDATED BALANCE SHEETS
| December 31, 2015 | December 31, 2014 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 132,229 | $ | 2,507,401 | ||||
| Restricted cash | 66,510 | 66,000 | ||||||
| Prepayment on acquisition | 3,744,517 | – | ||||||
| Prepaid expenses and other | 247,298 | 204,012 | ||||||
| Total current assets | 4,190,554 | 2,777,413 | ||||||
| Property and equipment, net | 62,021 | 119,449 | ||||||
| Goodwill | 3,283,379 | 3,465,157 | ||||||
| Indefinite-lived intangible assets | 9,243,128 | 9,754,857 | ||||||
| Other assets | 129,306 | 199,270 | ||||||
| Total assets | $ | 16,908,388 | $ | 16,316,146 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 1,788,521 | $ | 852,760 | ||||
| Accrued expenses | 1,487,046 | 1,409,691 | ||||||
| Hybrid debt instrument, net | 3,652,749 | – | ||||||
| Other current liabilities | 19,098 | 41,472 | ||||||
| Loans due to related parties | 395,000 | 395,000 | ||||||
| Total current liabilities | 7,342,414 | 2,698,923 | ||||||
| Deferred tax liability | 2,918,518 | 3,080,097 | ||||||
| Other liabilities | 38,791 | 56,383 | ||||||
| Total liabilities | 10,299,723 | 5,835,403 | ||||||
| Commitments and contingent liabilities (Note 14) | – | – | ||||||
| Stockholders' equity: | ||||||||
| Common stock, $0.001 par value; 45,454,546 and 6,528,970 shares authorized as of December 31, 2015 and December 31, 2014, respectively; 4,909,685 and 4,545,213 shares issued as of December 31, 2015 and December 31, 2014, respectively; 4,585,800 and 4,221,328 shares outstanding as of December 31, 2015 and December 31, 2014, respectively | 4,909 | 4,545 | ||||||
| Additional paid in capital | 99,763,101 | 90,806,130 | ||||||
| Accumulated deficit | (88,131,899 | ) | (75,624,428 | ) | ||||
| Accumulated other comprehensive income | 253,734 | 575,676 | ||||||
| Treasury stock | (5,281,180 | ) | (5,281,180 | ) | ||||
| Total stockholders' equity | 6,608,665 | 10,480,743 | ||||||
| Total liabilities and stockholders' equity | $ | 16,908,388 | $ | 16,316,146 | ||||
Note: All share and per share data have been adjusted to reflect the 1-for-33 reverse stock split which became effective June 1, 2016, as discussed in Note 2.
The accompanying notes are an integral part of these consolidated financial statements.
| F-14 |
XENETIC BIOSCIENCES, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
| YEAR ENDED DECEMBER 31, | ||||||||
| 2015 | 2014 | |||||||
| Operating costs and expenses: | ||||||||
| Research and development | $ | (3,434,016 | ) | $ | (6,323,896 | ) | ||
| General and administrative | (6,388,000 | ) | (6,600,870 | ) | ||||
| Loss from operations | (9,822,016 | ) | (12,924,766 | ) | ||||
| Other income (expense): | ||||||||
| Change in fair value of derivative liability | (2,125,117 | ) | – | |||||
| Loss on disposal of subsidiaries | – | (1,069,675 | ) | |||||
| Other expense | (295,033 | ) | (326,916 | ) | ||||
| Interest income | 1,694 | 18,959 | ||||||
| Interest expense | (266,999 | ) | (4,706 | ) | ||||
| (2,685,455 | ) | (1,382,338 | ) | |||||
| Net loss | (12,507,471 | ) | (14,307,104 | ) | ||||
| Other comprehensive loss from foreign currency translation adjustment | (321,942 | ) | (324,578 | ) | ||||
| Total comprehensive loss | $ | (12,829,413 | ) | $ | (14,631,682 | ) | ||
| Net loss per share of common stock, basic and diluted | $ | (3.02 | ) | $ | (3.55 | ) | ||
| Weighted-average shares of common stock outstanding, basic and diluted | 4,254,470 | 4,118,062 | ||||||
Note: All share and per share data have been adjusted to reflect the 1-for-33 reverse stock split which became effective June 1, 2016, as discussed in Note 2.
The accompanying notes are an integral part of these consolidated financial statements.
| F-15 |
XENETIC BIOSCIENCES, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| YEAR ENDED DECEMBER 31, | ||||||||
| 2015 | 2014 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (12,507,471 | ) | $ | (14,307,104 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 56,115 | 88,689 | ||||||
| Amortization of hybrid debt instrument discount | 108,527 | – | ||||||
| Non-cash interest expense | 153,791 | – | ||||||
| Share-based payments | 2,594,113 | 1,513,238 | ||||||
| Warrant expense for services | 933,195 | 239,889 | ||||||
| Change in fair value of derivative liability | 2,125,117 | – | ||||||
| Loss on issuance of hybrid debt instrument | 59,612 | – | ||||||
| Hybrid debt instrument issuance costs | (30,933 | ) | – | |||||
| Loss on disposal of subsidiaries | – | 1,069,675 | ||||||
| Fee paid on disposal of subsidiaries | – | (430,000 | ) | |||||
| Foreign currency translation | 369,947 | (353,952 | ) | |||||
| Other non-cash transactions | (127,875 | ) | – | |||||
| Changes in operating assets and liabilities: | ||||||||
| Other receivables, prepayments and other assets | 21,227 | (24,468 | ) | |||||
| Accounts payable, accrued expenses and other liabilities | 943,909 | (479,015 | ) | |||||
| Net cash used in operating activities | (5,300,726 | ) | (12,683,048 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchases of property and equipment | (1,663 | ) | (57,669 | ) | ||||
| Disposition of property and equipment | 7,882 | 5,487 | ||||||
| Cash acquired from acquisition | – | 43,502 | ||||||
| Cash transferred in connection with Hive Out Agreement | – | (43,502 | ) | |||||
| Net cash provided by (used in) investing activities | 6,219 | (52,182 | ) | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from issuance of debt | 3,100,000 | – | ||||||
| Payments on debt | (100,000 | ) | – | |||||
| Proceeds from issuance of common stock | – | 10,000,000 | ||||||
| Proceeds from exercise of stock options | – | 101,933 | ||||||
| Payments on loan from related party | – | (286,124 | ) | |||||
| Net cash provided by financing activities | 3,000,000 | 9,815,809 | ||||||
| Effect of exchange rate change on cash | (80,665 | ) | 587,336 | |||||
| Net change in cash, excluding restricted cash | (2,375,172 | ) | (2,332,085 | ) | ||||
| Cash at beginning of period | 2,507,401 | 4,839,486 | ||||||
| Cash at end of period | $ | 132,229 | $ | 2,507,401 | ||||
| SUPPLEMENTAL CASH FLOW INFORMATION: | ||||||||
| Cash paid for interest | $ | 592 | $ | 4,706 | ||||
| SUPPLEMENTAL SCHEDULE OF NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Interest paid in common stock | $ | 75,935 | $ | – | ||||
| Non-cash issuance of common stock in connection with pending asset acquisition | $ | 3,744,517 | $ | – | ||||
| Non-cash issuance of warrants in connection with debt | $ | 1,626,344 | $ | – | ||||
| Non-cash recording of derivative liability in connection with debt | $ | 1,419,105 | $ | – | ||||
| Equity consideration transferred in the acquisition | $ | – | $ | 3,750,000 | ||||
| Repurchase and cancellation of common stock in disposal of subsidiaries | $ | – | $ | (3,750,000 | ) | |||
The accompanying notes are an integral part of these consolidated financial statements.
| F-16 |
XENETIC BIOSCIENCES, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
| No. of shares of common stock | Ordinary shares | JSOP shares | Additional paid-in capital | Accumulated deficit | Accumulated other comprehensive income | Total shareholders’ equity | ||||||||||||||||||||||
| Par $0.001 | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||
| Balance as of January 1, 2014 | 3,957,032 | 3,957 | (5,281,180 | ) | 75,301,658 | (58,306,999 | ) | 900,254 | 12,617,690 | |||||||||||||||||||
| Exercise of stock options | 60,124 | 60 | 101,873 | 101,933 | ||||||||||||||||||||||||
| Issuance of common stock | 422,424 | 422 | 10,810,774 | 10,811,196 | ||||||||||||||||||||||||
| Issuance of warrants | 239,889 | 239,889 | ||||||||||||||||||||||||||
| Deemed issuance of shares in reverse merger | 409,091 | 409 | 3,749,591 | 3,750,000 | ||||||||||||||||||||||||
| Repurchase and cancellation of shares in Hive Out Agreement | (303,031 | ) | (303 | ) | (99,697 | ) | (3,010,325 | ) | (3,110,325 | ) | ||||||||||||||||||
| Repurchase and cancellation of shares in Acquisition | (427 | ) | (0 | ) | 0 | |||||||||||||||||||||||
| Share-based payments | 702,042 | 702,042 | ||||||||||||||||||||||||||
| Net loss | (14,307,104 | ) | (14,307,104 | ) | ||||||||||||||||||||||||
| Foreign currency translation | (324,578 | ) | (324,578 | ) | ||||||||||||||||||||||||
| Balance as of December 31, 2014 | 4,545,213 | 4,545 | (5,281,180 | ) | 90,806,130 | (75,624,428 | ) | 575,676 | 10,480,743 | |||||||||||||||||||
| Issuance of common stock | 31,138 | 31 | 337,309 | 337,340 | ||||||||||||||||||||||||
| Issuance of common stock in connection with pending asset acquisition | 333,334 | 333 | 3,744,184 | 3,744,517 | ||||||||||||||||||||||||
| Issuance of warrants | 933,195 | 933,195 | ||||||||||||||||||||||||||
| Issuance of warrants in connection with debt (net of issuance costs of $16,769) | 1,609,575 | 1,609,575 | ||||||||||||||||||||||||||
| Settlement of accrued interest in common stock | 75,935 | 75,935 | ||||||||||||||||||||||||||
| Share-based payments | 2,256,773 | 2,256,773 | ||||||||||||||||||||||||||
| Net loss | (12,507,471 | ) | (12,507,471 | ) | ||||||||||||||||||||||||
| Foreign currency translation | (321,942 | ) | (321,942 | ) | ||||||||||||||||||||||||
| Balance as of December 31, 2015 | 4,909,685 | 4,909 | (5,281,180 | ) | 99,763,101 | (88,131,899 | ) | 253,734 | 6,608,665 | |||||||||||||||||||
Note: All share and per share data have been adjusted to reflect the 1-for-33 reverse stock split which became effective June 1, 2016, as discussed in Note 2.
The accompanying notes are an integral part of these consolidated financial statements.
| F-17 |
XENETIC BIOSCIENCES, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 1. | The Company |
Background
Xenetic Biosciences, Inc. (the “Company”), incorporated in the state of Nevada and based in Lexington, Massachusetts, is a clinical stage biopharmaceutical company that is focused on the discovery, development and planned commercialization of a new generation of human drug therapies for the treatment of a variety of conditions including anemia, refractory acute myeloid leukemia, cystic fibrosis and certain other cancers based upon its proprietary and patented drug delivery platform systems and drug development collaborations with major third party pharmaceutical companies around the world.
The Company’s core technologies include PolyXen™ a platform for creating next generation biologic drugs by extending the efficacy, safety and half-life of existing biologic drugs, OncoHist™, for the development of novel oncology drug therapies focused on orphan indications in humans, and ImuXen™, for the development of vaccines that can simultaneously deliver multiple active pharmaceutical ingredients. The Company is also developing a broad pipeline of drug candidates for next generation biologics and novel oncology therapeutics in a number of orphan disease indications.
Going Concern and Management’s Plan
While these consolidated financial statements have been prepared on a going concern basis, if the Company does not successfully raise additional working capital, there can be no assurance that the Company will be able to continue its operations and these conditions raise substantial doubt about its ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments related to the recoverability or classification of asset-carrying amounts or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
In March 2016, the Company engaged an investment banking firm to assist with a proposed sale of the Company’s securities. The Company is optimistic that it will be successful in obtaining financing, however there can be no assurance that it will be able to do so or, if it is able to, that is can do so under commercially reasonable terms. In the event the Company is unsuccessful in this proposed sale, the Company will plan to rely upon proceeds from the sale of up to $6.5 million in securities to OJSC Pharmsynthez (“Pharmsynthez”) as provided for in the AS Kevelt Asset Purchase Agreement.
AS Kevelt Asset Purchase Agreement
In November 2015, the Company entered into an Asset Purchase Agreement (the “APA”) with AS Kevelt, an Estonian company (“Kevelt”), and Pharmsynthez, parent of Kevelt (together referred to as the “Sellers”). Pursuant to the APA, Kevelt will transfer to the Company certain intellectual property rights held by the Sellers with respect to the immunomodulatory product candidate Virexxa® held by Kevelt and the Sellers will grant the Company the worldwide right to develop, market and license Virexxa® for certain uses, except for excluded uses in Russia, the Commonwealth of Independent States and certain other countries. In consideration, the Company will issue to Pharmsynthez 3,045,455 shares of its common stock. The APA also provides for the Company’s issuance of 10% Senior Secured Convertible Promissory Notes of up to $3.5 million to Pharmsynthez (the “APA Notes”) and certain warrants to purchase a number of shares equal to the issuable shares of the Company’s stock upon conversion of the APA Notes. There is also a provision in the APA for the contingent sale of up to $6.5 million of the Company’s common stock in the event of a qualifying capital raise. Also as part of the APA, Dr. Dmitry Genkin and Kirill Surkhov, shareholders and founders of Pharmsynthez, will assign a U.S. provisional patent application to the Company in exchange for 333,334 shares of the Company’s common stock. The Company issued 333,334 shares in November 2015 as a prepayment toward completing the APA transaction, recording $3.74 million as the proportional fair value of the total consideration to be recorded upon the completion of the APA transaction.
In connection with the APA, certain terms in the Securities Purchase Agreement (the “SPA”) with Pharmsynthez issued in July 2015 were modified. See Note 8, Hybrid Debt Instrument, for further discussion of the SPA. As of December 31, 2015, the APA was not yet consummated and is contingent upon the parties meeting their respective closing conditions as set forth in the APA. The APA transaction is expected to be completed during 2016.
2014 Business Combination
On January 23, 2014, the Company consummated a reverse merger (the “Acquisition”) pursuant to a written plan of reorganization, in which the Company merged with Xenetic Biosciences (UK) Limited (formerly Xenetic Biosciences plc) (“Xenetic UK”), a company incorporated in England and Wales under the Companies Act of 1985, such that Xenetic UK became a wholly owned subsidiary of the Company. Upon completion of the Acquisition, the Company acquired all issued and outstanding shares of capital stock of Xenetic UK. As a result 4,016,531 shares of the Company’s common stock were newly issued and, immediately following the Acquisition, there were 4,122,592 shares of common stock issued and outstanding. At that time, because former Xenetic UK shareholders owned approximately 97% of the combined company on a fully diluted basis and all members of the combined company’s executive management were from Xenetic UK, Xenetic UK was deemed to be the acquiring company for accounting purposes and the transaction was accounted for as a reverse acquisition in accordance with accounting principles generally accepted in the United States (“US GAAP”).
| F-18 |
Prior to the Acquisition, the Company changed its name from General Sales and Leasing, Inc. to Xenetic Biosciences, Inc. As used in these consolidated financial statements, unless otherwise indicated, all references herein to “Xenetic”, the “Company”, “we” or “us” refer to Xenetic Biosciences, Inc. and its wholly owned subsidiaries.
| 2. | Summary of Significant Accounting Policies |
Preparation of Financial Statements
These consolidated financial statements have been prepared on the assumption that the Company will be able to realize its assets and discharge its liabilities in the normal course of business. This assumption is presently in question and contingent upon the Company’s ability to raise additional working capital. The financial statements do not include any adjustments relating to recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
Certain prior period amounts have been reclassified to conform to the presentation for the current period.
On June 1, 2016, the Company effected a reduction, on a 1 for 33 basis, in our authorized common stock, par value $0.001, along with a corresponding and proportional decrease in the number of shares issued and outstanding. On the effective date of the reverse stock split, (i) every 33 shares of common stock were reduced to one share of common stock; (ii) the number of shares of common stock into which each outstanding warrant or option to purchase common stock were proportionately reduced on the same basis as the common stock; and (iii) the exercise price of each outstanding warrant or option to purchase common stock were proportionately increased on a 1-to-33 basis. All of the share numbers, share prices, and exercise prices have been adjusted, on a retroactive basis, to reflect this 1-for-33 reverse stock split.
Principles of Consolidation
The financial statements of the Company include the accounts of Xenetic UK and its wholly owned subsidiaries: Lipoxen Technologies Limited (“Lipoxen”), Xenetic Bioscience, Incorporated, and SymbioTec GmbH (“SymbioTec”). All material intercompany balances and transactions have been eliminated on consolidation.
Use of Estimates
The consolidated financial statements and accompanying notes are prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). The preparation of the financial statements in accordance with US GAAP requires management to make estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, the reported amounts of revenue and expenses in the financial statements and disclosures in the accompanying notes. Actual results and outcomes may differ materially from management’s estimates, judgments and assumptions.
Change in Accounting Principle
During the second quarter of 2015, the Company elected to apply pushdown accounting to the Company’s acquisition of SymbioTec that occurred in 2012. Pushdown accounting refers to the use of the acquirer’s basis in the preparation of the acquiree’s separate financial statements as the new basis of accounting for the acquiree. Application of pushdown accounting is treated as a change in accounting principle and was applied retrospectively to the Company’s consolidated financial statements. This change resulted in no impact to the consolidated financial statements for the year ended December 31, 2015 or 2014.
Functional Currency Change
Effective April 1, 2015, the functional currency of the Company’s foreign subsidiaries changed from the British Pound Sterling to the United States (“U.S.”) dollar. The changes in the economic facts and circumstances that caused the functional currency to change to that of the parent company include: the closing of the Company’s last office outside of the U.S. during the first quarter of 2015, a shift of financial dependence of the subsidiaries to the parent and the growth of the Company’s operations in U.S. dollar-denominated expenses. The Company translated assets and liabilities of these foreign subsidiaries at the exchange rate in effect at the balance sheet date and included accumulated net translation adjustments in equity as a component of accumulated other comprehensive loss. The change in functional currency is applied on a prospective basis. Therefore, any gains and losses that were previously recorded in accumulated other comprehensive loss remain unchanged through March 31, 2015. Foreign currency transaction gains and losses are the result of exchange rate changes on transactions denominated in currencies other than the functional currency. The remeasurement of those foreign currency transactions is included in determining net income or loss for the period of exchange.
| F-19 |
Foreign Currency Translation
The Company’s reporting currency is U.S. dollars. During the years ended December 31, 2015 and 2014, the Company had operations in the U.S., United Kingdom (“U.K.”) and Germany. Assets and liabilities of foreign operations were translated to U.S. dollars at the exchange rate in effect at the balance sheet date and revenue and expenses at the average exchange rate for the period. Gains and losses from the translation of the consolidated financial statements of foreign subsidiaries into U.S. dollars were included in stockholders’ equity as a component of other comprehensive income. The Company did not record tax provisions or benefits for the net changes in foreign currency translation adjustments, as the Company intends to permanently reinvest undistributed earnings in its foreign subsidiaries. Following the change in the functional currency of the Company’s foreign subsidiaries to the U.S. dollar on April 1, 2015, it is no longer necessary to record gains and losses from the translation of the consolidated financial statements of foreign subsidiaries from a foreign functional currency into the reporting currency.
Realized and unrealized gains and losses resulting from foreign currency transactions arising from exchange rate fluctuations on balances denominated in currencies other than the functional currencies, are recognized in “Other (expense) income” in the consolidated statements of comprehensive loss. Monetary assets and liabilities that are denominated in a currency other than the functional currency are re-measured to the functional currency using the exchange rate at the balance sheet date and gains or losses are recorded within the “Other income (expense)” section of the consolidated statements of comprehensive loss.
Correction of Identified Errors
During the second quarter of 2015, the Company identified an error in the consolidated financial statements related to the accounting for foreign currency matters. One of the Company’s subsidiary’s functional currency had been incorrectly designated as the Euro instead of British Pound Sterling during the period January 1, 2013 through March 31, 2015. As a result, certain applicable financial results of this entity were being translated to the reporting currency when they should have been first remeasured into the functional currency. In addition, the Company identified an error in the consolidated financial statements related to the pushdown accounting of that subsidiary. The new basis of accounting of the acquired entity formed as a result of the acquisition was not first remeasured into the functional currency before being translated to the reporting currency.
The correction of the errors identified above resulted in the recognition of foreign currency net gains and foreign currency translation net losses. We concluded that these adjustments were not material to the Company’s financial position or results of operations for any of the prior periods presented. Therefore, we recognized the cumulative impact during the three months ended June 30, 2015, which resulted in a net gain in other income (expenses) in the consolidated statement of comprehensive loss of $0.24 million for the year ended December 31, 2015 and a cumulative impact in accumulated other comprehensive income in the consolidated balance sheet of $0.31 million as of June 30, 2015.
Fair Value of Financial Instruments
ASC Topic 820 Fair Value Measurement defines fair value as the price that would be received to sell an asset or be paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Company applies the following fair value hierarchy, which prioritizes the inputs used to measure fair value into three levels and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement. Level 1 inputs are quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. Level 2 utilizes quoted market prices in markets that are not active, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency. Level 3 inputs are unobservable inputs for the asset or liability in which there is little, if any, market activity for the asset or liability at the measurement date. See Note 9, Fair Value Measurements, for discussion of the Company’s fair value measurements.
Cash, Cash Equivalents and Investments
The Company considers all highly liquid investments with maturities of 90 days or less from the date of purchase to be cash equivalents. Investments with original maturities of greater than 90 days from the date of purchase but less than one year from the balance sheet date are classified as short-term investments, while investments with maturities of one year or beyond from the balance sheet date are classified as long-term investments. Management determines the appropriate classification of its cash equivalents and investment securities at the time of purchase and re-evaluates such determination as of each balance sheet date.
| F-20 |
Restricted Cash
As of December 31, 2015 and 2014, restricted cash represents a certificate of deposit that matures annually, and secures the Company’s outstanding letter of credit of $66,000 for the operating lease for office and laboratory space in Lexington, Massachusetts. The letter of credit is required to be maintained through the term of the lease, which expires in January 2019.
Concentration of Credit Risk
Financial instruments that subject the Company to concentrations of credit risk include cash and cash equivalents. The Company maintains cash and cash equivalents with various major financial institutions. The Company performs periodic evaluations of the relative credit standing of these financial institutions and limits the amount of credit exposure with any one institution.
Property and Equipment
The Company records property and equipment at cost less accumulated depreciation. Expenditures for major renewals and improvements which extend the life or usefulness of the asset are capitalized. Items of an ordinary repair or maintenance nature are charged directly to operating expense as incurred. The Company calculates depreciation using the straight-line method over the estimated useful lives of the assets:
| Asset Classification | Estimated Useful Life | |
| Laboratory equipment | 3 years | |
| Office and computer equipment | 3 years | |
| Leasehold improvements | 5 years or the remaining term of the lease, if shorter | |
| Furniture and fixtures | 5 years |
The Company eliminates the cost of assets retired or otherwise disposed of, along with the corresponding accumulated depreciation, from the related accounts, and the resulting gain or loss is reflected in the results of operations.
Indefinite-Lived Intangible Assets
Acquired indefinite-lived intangible assets consist of In-Process Research and Development (“IPR&D”) related to the Company’s business combination with SymbioTec, which was recorded at fair value on the acquisition date. IPR&D intangible assets are considered indefinite-lived intangible assets until completion or abandonment of the associated research and development efforts. Substantial additional research and development may be required before the Company’s IPR&D reaches technological feasibility. Upon completion of the IPR&D project, the IPR&D assets will be amortized over their estimated useful lives.
In accordance with ASC Topic 350, Intangibles - Goodwill and Other (“ASC 350”), the Company assesses intangible assets with indefinite lives for impairment at least annually as of October 1, or when events or changes in the business environment indicate the carrying value may not be fully recoverable. The Company also has the option to first assess qualitative factors to determine whether the existence of events or circumstances leads to the determination that it is more likely than not (that is, a likelihood of more than 50%) that the acquired IPR&D is impaired. If the Company chooses to first assess the qualitative factors and it is determined that it is not more likely than not acquired IPR&D is impaired, the Company is not required to take further action to test for impairment. The Company also has the option to bypass the qualitative assessment and perform only the quantitative impairment test, which the Company may choose to perform in some periods but not in others.
No impairment was recorded during the years ended December 31, 2015 and 2014.
| F-21 |
Goodwill
Goodwill is comprised of the purchase price of business combinations in excess of the fair value assigned at acquisition to the net tangible and identifiable intangible assets acquired. Goodwill is not amortized, but in accordance with ASC 350, the Company assesses goodwill for impairment at least annually, or when events or changes in the business environment indicate the carrying value may not be fully recoverable. The Company also has the option to first assess qualitative factors to determine whether the existence of events or circumstances leads to the determination that it is more likely than not (that is, a likelihood of more than 50%) that goodwill is impaired. If the Company chooses to first assess qualitative factors and it is determined that it is not more likely than not goodwill is impaired, the Company is not required to take further action to test for impairment. The Company also has the option to bypass the qualitative assessment and perform only the quantitative impairment test, which the Company may choose to do in some periods but not in others. The Company performs its annual impairment review as of October 1.
No impairment was recorded during the years ended December 31, 2015 and 2014.
Impairment of Long-Lived Assets
In accordance with ASC Topic 360 Property, Plant and Equipment, the Company reviews long-lived assets to be held and used, including property and equipment, for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets or asset group may not be fully recoverable. No such impairments were recorded during the years ended December 31, 2015 and 2014.
Evaluation of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset or asset group and its eventual disposition. Impairment, if any, is calculated as the amount by which an asset’s carrying value exceeds its fair value, typically using discounted cash flows to determine fair value.
Embedded Derivatives Related to Debt Instruments
Embedded derivatives that are required to be bifurcated from their host contract are evaluated and valued separately from the host contract (i.e., the debt instrument). Features of the Company’s debt instrument that meet the definition of a derivative and the criteria for separate accounting include the conversion feature and certain put options. Embedded derivatives are valued individually and recorded as a compound derivative. The compound derivative is presented together with the host debt instrument and the related debt discount on a combined basis. Changes in the estimated fair value of the bifurcated embedded derivatives are reported as gains and losses in the consolidated statement of comprehensive loss each reporting period.
Revenue Recognition
The Company enters into supply, license and collaboration arrangements with pharmaceutical and biotechnology partners, some of which include royalty agreements based on potential net sales of approved commercial pharmaceutical products. The Company recognizes revenue in accordance with the authoritative guidance, ASC Topic 605, Revenue Recognition. The Company recognizes revenue when all of the following criteria are met: (i) persuasive evidence of an arrangement exists, (ii) delivery (or passage of title) has occurred or services have been rendered, (iii) the seller’s price to the buyer is fixed or determinable, and (iv) collectability is reasonably assured.
The terms of the Company’s license agreements include delivery of an Intellectual Property (“IP”) license to a collaboration partner. The Company may be compensated under license arrangements through a combination of non-refundable upfront payments, development and regulatory objective payments and royalty payments on future product sales by partners. Non-refundable upfront payments and development and regulatory objective payments received by the Company in license and collaboration arrangements that include future obligations, such as supply obligations, are recognized ratably over the Company’s expected performance period under each respective arrangement. The Company makes its best estimate of the period over which the Company expects to fulfil the Company’s performance obligations, which may include technology transfer assistance, research activities, clinical development activities, and manufacturing activities from development through the commercialization of the product. Given the uncertainties of these collaboration arrangements, significant judgment is required to determine the duration of the performance period. Non-refundable upfront license fees received, whereby continued performance or future obligations are considered inconsequential or perfunctory to the relevant licensed technology, are recognized as revenue upon delivery of the technology.
| F-22 |
The Company expects to recognize royalty revenue in the period of sale, based on the underlying contract terms, provided that the reported sales are reliably measurable and the Company has no remaining performance obligations, assuming all other revenue recognition criteria are met.
Reimbursements for research and development services completed by the Company related to the collaboration agreements are recognized in operations as revenue on a gross basis.
The Company’s license and collaboration agreements with certain collaboration partners could also provide for future payments to the Company based solely upon the performance of the respective collaboration partner in consideration of deadline extensions or upon the achievement of specified sales volumes of approved drugs. For such payments, the Company expects to recognize the payments as revenue when earned under the applicable contract terms on a performance basis or ratably over the term of the agreement. These payments may also be recognized as revenue when continued performance or future obligations by the Company are considered inconsequential or perfunctory.
Research and Development Expenses
Research and development expenses consist of expenses incurred in performing research and development activities, including compensation and benefits, facilities expenses, overhead expenses, clinical trial and related clinical manufacturing expenses, fees paid to clinical research organizations and clinical manufacturing organizations and other outside expenses. The Company expenses research and development costs as incurred. The Company expenses upfront, non-refundable payments made for research and development services as obligations are incurred. The value ascribed to intangible assets acquired but which have not met capitalization criteria is expensed as research and development at the time of acquisition.
Share-based Payments
Stock options
The Company grants share-based payments in the form of options to employees and nonemployees, Joint Share Ownership Plan (“JSOP”) awards to employees, as well as agreements to issue common stock in exchange for services provided by nonemployees. The Company measures share-based payments to employees in accordance with ASC Topic 718, Compensation – Stock Compensation and to nonemployees in accordance with ASC Topic 505, Equity.
Stock option compensation expenses are based on the fair value of the option calculated using the Black-Scholes option pricing model. Determining the appropriate fair value model and related assumptions requires judgment, including estimating share price volatility and expected terms of the awards. The expected volatility rates are estimated based on the actual volatility of the Company and of comparable public companies over the expected term. The expected terms represent the time that options are expected to be outstanding. The Company estimates forfeitures at the time of grant and revises those estimates in subsequent periods if actual forfeitures differ from those estimates. The Company has not paid dividends and does not anticipate paying cash dividends in the foreseeable future and, accordingly, uses an expected dividend yield of zero. The risk-free interest rate is based on the rate of US Treasury securities with maturities consistent with the estimated expected term of the awards. Upon exercise, stock options are redeemed for newly issued shares of common stock.
For employee options that vest based solely on service conditions, the fair value measurement date is generally on the date of grant and the related compensation expense, less expense for expected forfeitures, is recognized on a straight-line basis over the requisite vesting period of the awards.
For nonemployee options, the fair value measurement date is the earlier of the date the performance of services is complete or the date the performance commitment has been reached. The Company generally determines that the fair value of the stock options is more reliably measurable than the fair value of the services received. Compensation expense related to stock options granted to nonemployees that vest based solely on service conditions is subject to re-measurement at each reporting period until the options vest and is recognized on a straight-line basis over the requisite vesting period of the awards.
Common stock awards
The Company grants common stock awards to nonemployees in exchange for services provided. The Company generally measures the fair value of these awards using the fair value of the services provided as it is a more reliable measure of the fair value of the awards. The fair value measurement date of these awards is generally the date the performance of services is complete. The fair value of the awards is recognized on a straight-line basis as services are rendered. The share-based payments related to common stock awards for the settlement of services provided by nonemployees is recorded on the consolidated statement of comprehensive loss in the same manner and charged to the same account as if such settlements had been made in cash.
| F-23 |
Joint Share Ownership Plan awards
The Company measures the fair value of JSOP awards using Monte Carlo simulations based on the terms of the plan, which includes vesting conditions based on the achievement of certain market conditions in the form of share price hurdles. Determination of the appropriate fair value model and related assumptions requires judgment, including estimating share price volatility and the expected term of the awards. Accordingly, the Company recognizes compensation expense related to its JSOP awards using a graded vesting model.
Warrants
In connection with certain financing, consulting and collaboration arrangements, the Company issues warrants to purchase shares of its common stock. The outstanding warrants are standalone instruments that are not puttable or mandatorily redeemable by the holder and are classified as equity awards. The Company measures the fair value of the awards using the Black-Scholes option pricing model as of the measurement date. Warrants issued to collaboration partners in conjunction with the issuance of common stock are initally recorded at fair value as a reduction in additional paid-in capital of the common stock issued.
All other warrants are recorded at fair value as expense over the requisite service period or at the date of issuance, if there is not a service period. Warrants granted in connection with ongoing arrangements are more fully described in Note 11, Stockholders’ Equity.
Income Taxes
The Company accounts for income taxes using the liability method in accordance with ASC Topic 740, Income Taxes. Under this method, deferred tax assets and liabilities are determined based on temporary differences resulting from the different treatment of items for tax and financial reporting purposes. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to reverse. Additionally, the Company must assess the likelihood that deferred tax assets will be recovered as deductions from future taxable income. The Company evaluates the recoverability of its deferred tax assets on a quarterly basis.
Basic and Diluted Net Loss per Share
The Company computes basic net loss per share by dividing net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding during the period. The Company computes diluted net loss per share after giving consideration to the dilutive effect of stock options that are outstanding during the period, except where such non-participating securities would be anti-dilutive. The Company’s JSOP awards, prior to exercise, are considered treasury shares by the Company and thus do not impact the Company’s net loss per share calculation. As of December 31, 2015 and 2014, there were 323,885 JSOP awards issued.
Basic and diluted net loss per share are the same for the years ended December 31, 2015 and 2014 as the Company was in a net loss position. Potentially dilutive non-participating securities have not been included in the calculations of diluted net loss per share, as their inclusion would be anti-dilutive. As of December 31, 2015 and 2014, approximately 365,000 and 360,000 potentially dilutive securities were deemed anti-dilutive.
Segment Information
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, who is the Company’s Chief Executive Officer, in making decisions on how to allocate resources and assess performance. The Company views its operations and manages its business in one operating segment.
| F-24 |
Operating Leases
The Company leases an administrative and laboratory facility under an operating lease. Lease agreements may include rent holidays, rent escalation clauses and tenant improvement allowances. The Company recognizes scheduled rent increases on a straight-line basis over the lease term beginning with the date the Company takes possession of the leased space.
Acquisitions
The Company has a history of engaging in acquisition transactions that require the Company to evaluate whether the transaction meets the criteria of a business combination and, in some cases, whether it meets the definition of a reverse merger. For those acquisitions that meet the criteria for a reverse merger, the Company evaluates the entities involved to distinguish the appropriate accounting acquirer and acquiree according to ASC 805. If the transaction does not meet the business combination requirements, the transaction is accounted for as an asset acquisition or recapitalization and no goodwill is recognized. If the acquisition meets the definition of a business combination, the Company allocates the purchase price, including any contingent consideration, to the assets acquired and the liabilities assumed at their estimated fair values as of the date of the acquisition with any excess of the purchase price paid over the estimated fair value of net assets acquired recorded as goodwill. The fair value of the assets acquired and liabilities assumed is typically determined by using either estimates of replacement costs or discounted cash flow valuation methods.
When determining the fair value of tangible assets acquired, the Company estimates the cost to replace the asset with a new asset, taking into consideration such factors as age, condition and the economic useful life of the asset. When determining the fair value of intangible assets acquired, the Company uses judgment to estimate the applicable discount rate, growth rates and the timing and amount of future cash flows. The fair value of assets acquired and liabilities assumed is typically determined using the assistance of an independent third party specialist.
Business combination related costs are expensed in the period in which the costs are incurred and the services are received. Asset acquisition related costs are generally capitalized as a component of cost of the assets acquired.
Recent Accounting Pronouncements
In March 2016, Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-06, Derivatives and Hedging (Topic 815) (“ASU 2016-06”). ASU 2016-06 clarifies the requirements for assessing whether contingent call or put options that can accelerate the payment of principal on debt instruments are clearly and closely related to their debt hosts. This guidance is effective for annual reporting periods beginning after December 15, 2016, including interim periods within those annual periods. Early application is permitted. The Company is currently evaluating the impact of this new standard.
In February 2016, FASB issued ASU 2016-02, Leases (Topic 842) (“ASU 2016-02”). ASU 2016-02 will require lessees to recognize a lease liability and a right-of-use asset for all leases, with the exception of short-term leases, at the commencement date. This guidance is effective for annual reporting periods beginning after December 15, 2018, including interim periods within those annual periods. Early application is permitted. The Company is currently evaluating the impact of this new standard.
| F-25 |
In November 2015, FASB issued ASU 2015-17, Income Taxes (Topic 740) (“ASU 2015-17”). ASU 2015-17 simplifies the presentation of deferred income taxes by requiring that deferred tax assets and liabilities be classified as non-current in a classified statement of financial position. This guidance is effective for annual reporting periods beginning after December 15, 2016, including interim periods within those annual periods, with early adoption permitted. The Company early adopted ASU 2015-17 for the year ended December 31, 2015 on a prospective basis, as permitted.
In April 2015, FASB issued ASU 2015-03, Interest – Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs (“ASU 2015-03”). ASU 2015-03 requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. This guidance is effective for annual reporting periods beginning after December 15, 2015, and interim periods within fiscal years beginning after December 15, 2016, with early adoption permitted. The Company early adopted ASU 2015-03 in July 2015, as permitted. There was no impact of early adoption of ASU 2015-03 on the Company’s consolidated financial statements previously reported.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements – Going Concern (Subtopic 205-40) (“ASU 2014-15”). ASU 2014-15 defines management’s responsibility to evaluate whether there is substantial doubt about an organization’s ability to continue as a going concern and provides guidance on the related footnote disclosures. This guidance is effective for annual reporting periods beginning after December 15, 2016, and interim periods within annual periods beginning after December 15, 2016. Early application is permitted. The Company is currently evaluating the impact of this new standard.
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers (Topic 606) (“ASU 2014-09”). ASU 2014-09 supersedes the revenue recognition requirements in Accounting Standards Codification (“ASC”) Topic 605, Revenue Recognition, and most industry-specific guidance. The core principle of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods or services. In August 2015, the FASB issued ASU 2015-15, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date, which defers the effective date of ASU 2014-09 for all entities by one year. This guidance is currently effective for annual reporting periods beginning after December 15, 2017, including interim periods within that reporting period, under either full or modified retrospective approach. Early application is permitted as of annual reporting periods beginning after December 15, 2016. The Company is currently evaluating the impact of this new standard on its revenue recognition policy.
The Company has considered other recent accounting pronouncements and concluded that they are either not applicable to the business, or that no material effect is expected on the consolidated financial statements as a result of future adoption.
| 3. | Acquisitions |
2014 Business Combination
On January 23, 2014, the Company completed the Acquisition transaction with Xenetic UK which resulted in the Company acquiring all of the issued and outstanding common stock of Xenetic UK. The Acquisition was accounted for as a reverse acquisition under the acquisition method of accounting per ASC 805, with Xenetic UK treated as the accounting acquirer and the Company treated as the “acquired” company for financial reporting purposes. This was determined based on the following facts: (i) after the reverse merger, former shareholders of Xenetic UK held a majority of the voting interest of the combined company; (ii) former Board of Directors of Xenetic UK possess majority control of the Board of Directors of the combined company; and (iii) members of the management of Xenetic UK are responsible for the management of the combined company. As such, the financial statements of Xenetic UK are treated as the historical financial statements of the combined company.
The fair value of the consideration transferred in the reverse merger was $3.75 million. This was calculated as the number of shares of common stock that Xenetic UK would have had to issue in order for the Company’s shareholders to hold the same equity interest in the combined entity immediately following the acquisition (approximately 9.2%), multiplied by the estimated fair value of the Company’s common stock on the acquisition date (£1.98 per share). The estimated fair value of the Company’s common stock was based on the price of the Company’s stock on the acquisition date, which was actively traded on the Alternative Investments Market of the London Stock Exchange in the United Kingdom. In addition, Xenetic UK incurred approximately $3 million of transaction costs related to the reverse merger. The Company recognized approximately $0.5 million of transaction costs related to the reverse merger in general and administrative expenses on the consolidated statement of comprehensive loss during the year ended December 31, 2014. No transaction costs related to the reverse merger were recognized during the year ended December 31, 2015.
| F-26 |
As of December 31, 2014, the Company finalized the purchase accounting for the Acquisition. Management determined the purchase price allocations based on estimates of the fair values of all assets acquired and liabilities assumed. The Company believe that such information provides a reasonable basis for estimating the fair values of assets acquired and liabilities assumed. The fair values of the acquired assets and liabilities assumed are as follows:
| Cash | $ | 43,502 | ||
| Accounts receivable | 145 | |||
| Prepaid expenses | 8,643 | |||
| Property, plant and equipment | 331,500 | |||
| Accounts payable | (354,079 | ) | ||
| Accrued expenses | (36,146 | ) | ||
| Long-term debt | (372,813 | ) | ||
| Total identifiable net assets | (379,248 | ) | ||
| Goodwill | 4,129,248 | |||
| Total | $ | 3,750,000 |
Following the Acquisition, an Agreement of Conveyance, Transfer and Assignment of Subsidiaries and Assumption of Obligations (the “Hive Out Agreement”) was executed, whereupon 303,031 outstanding shares of common stock held by Oxbridge Technology Partners SA (“Oxbridge”) were returned to the Company and recorded as treasury shares and were subsequently canceled. In exchange, Oxbridge acquired all issued and outstanding shares of both of the Company’s former operating subsidiaries, Shift It Media Co. and General Aircraft, Inc. (the “Disposed Subsidiaries”), including all assets and liabilities connected with the businesses transferred. In addition, the Company disposed of the associated goodwill. The Hive Out Agreement also required a payment to Oxbridge of $430,000, which was paid by the Company shortly after the Acquisition.
The Company recorded this divestiture as a separate transaction from the Acquisition that results in the disposal of two of the Company’s subsidiaries. The Disposed Subsidiaries did not record any operations in the combined entity following the Acquisition before they were disposed and these financial statements do not reflect the historical financial statements of the Disposed Subsidiaries as they were previously owned by the accounting acquiree. Accordingly, there are no balances to be recorded as discontinued operations on the statement of comprehensive loss. As a result of the divestiture of the Disposed Subsidiaries, the Company recorded a loss on disposal of subsidiaries of $1,069,675 during the year ended December 31, 2014.
Due to the nature of the Acquisition and related Hive Out Agreement, the transaction did not result in any adjustments with a continuing impact on the Company’s results of operations.
2015 Asset Purchase Agreement
In November 2015, the Company entered into the APA with Kevelt and Pharmsynthez, parent of Kevelt. Pursuant to the APA, the Sellers will transfer to the Company certain intellectual property rights held by the Sellers with respect to the immunomodulatory product candidate Virexxa® held by Kevelt and the Sellers will grant the Company the worldwide right to develop, market and license Virexxa® for certain uses except for excluded uses in Russia, the Commonwealth of Independent States and certain other countries. In consideration, the Company will issue to Pharmsynthez 3,045,455 shares of the Company’s common stock. Also as part of the APA, Dr. Dmitry Genkin and Kirill Surkhov, shareholders and founders of Pharmsynthez, will assign a U.S. provisional patent application to the Company in exchange for 333,334 shares of the Company’s common stock.
During December 2015, the 333,334 shares were issued to Dr. Genkin and Mr. Surkhov under the terms of the APA. However, as of December 31, 2015, the APA transaction was not yet consummated and is contingent upon the parties meeting their respective closing conditions as set forth in the APA. As a result, the Company recorded approximately $3.74 million, the fair value of the proportional consideration provided, as a prepayment within current assets on the consolidated balance sheet as of December 31, 2015.
The APA also provides for the Company’s issuance of 10% Senior Secured Convertible Promissory Notes of up to $3.5 million to Pharmsynthez and certain warrants to purchase shares of the Company’s common stock. In connection with the APA, certain terms in the SPA with Pharmsynthez issued in July 2015 were modified. See Note 8, Hybrid Debt Instrument, for discussion of the SPA and Note 11, Stockholders’ Equity, for discussion of the warrants.
There is also a provision in the APA for the contingent sale of up to $6.5 million of the Company’s common stock in the event of a qualifying capital raise.
The APA transaction is expected to be completed during 2016.
| F-27 |
| 4. | Significant Strategic Drug Development Collaborations - Related Parties |
Baxalta Incorporated
In August 2005, the Company entered into an exclusive research, development, license and supply agreement with Baxter Healthcare SA (“Baxter SA”) and Baxter Healthcare Corporation (together referred to as “Baxter”) to develop products with an extended half-life of certain proteins and molecules using the Company’s patent protected PolyXen™ technology whereby polysialic acid (“PSA” – a chain of polysialic acids) is conjugated with Baxter’s proprietary molecule(s) to create a new generation of drugs to treat the failure of blood to coagulate in the therapeutic treatment of blood and bleeding disorders, such as hemophilia. The lead candidate in this collaboration is a longer-acting form of a recombinant Factor VIII (“rFVIII”) protein. During June 2015, in connection with the separation of its biopharmaceuticals business to form Baxalta Incorporated (“Baxalta”), Baxter assigned all of its rights and obligations under its existing agreement with the Company to Baxalta.
This agreement has been amended several times since 2005, most recently in January 2014. The January 2014 amendment provides for increased future development, regulatory, sales and deadline extension receipts, restructured target deadlines and royalty receipts on potential net sales. The Company is entitled to up to $100 million in potential development, regulatory, sales and deadline extension receipts, which are contingent on the performance of Baxalta achieving certain milestones. The Company is also entitled to royalties on potential net sales varying by country of sale. The Company’s right to receive these royalties in any particular country will expire upon the later of ten years after the first commercial sale of the product in that country or the expiration of patent rights in that particular country. In connection with this amendment, Baxter SA also made a $10 million equity investment in the Company in exchange for 324,097 shares of the Company’s common stock during January 2014.
Through December 31, 2015, the Company and Baxalta continued to engage in research and development activities with no resultant commercial products. The Company did not recognize revenue in connection with this collaboration during the years ended December 31, 2015 and 2014.
Baxalta is a related party of the Company, with a share ownership of approximately 8.0% and 8.7% of the total issued common stock of the Company as of December 31, 2015 and 2014, respectively.
SynBio LLC
In August, 2011, SynBio LLC (“SynBio”) and the Company entered into a stock subscription and collaborative development of pharmaceutical products agreement (the “Co-Development Agreement”). The Company granted an exclusive license to SynBio to develop pharmaceutical products using certain molecule(s) based on SynBio’s technology and the Company’s proprietary technology (PolyXen™, OncoHist™ and ImuXen™) that prolongs the active life and/or improves the pharmacokinetics of certain therapeutic proteins and peptides (as well as conventional drugs). In return, SynBio granted an exclusive license to the Company to use the pre-clinical and clinical data generated by SynBio in certain agreed products and engage in the development of commercial candidates.
SynBio and the Company are each responsible for funding their own research activities. There are no milestone or other research-related payments due under the agreement other than fees for the supply of each company’s respective research supplies based on their technology, which, when provided, are due to mutual convenience and not representative of an ongoing or recurring obligation to supply research supplies. Most recently, similar to the Company’s agreement with Baxalta, Serum Institute of India Limited (“Serum Institute”) has agreed to directly provide the research supplies to SynBio, where the Company is not liable for any failure to supply the research supplies as a result of any act or fault of Serum Institute’s. Upon successful commercialization of any resultant products, the Company is entitled to receive royalties on sales in certain territories and pay royalties to SynBio for sales outside those certain territories.
| F-28 |
Through December 31, 2015, the Company and SynBio continued to engage in research and development activities with no resultant commercial products. The Company did not recognize revenue in connection with the Co-Development Agreement during the years ended December 31, 2015 and 2014.
SynBio is an affiliate of the Company, with a share ownership of approximately 39.0% and 41.6% of the total issued common stock of the Company as of December 31, 2015 and 2014, respectively. On December 31, 2014, the Company granted SynBio a warrant to purchase 204,394 shares of common stock in connection with ongoing collaborative activities. See Note 9, Stockholders’ Equity, for further information on the warrant.
Serum Institute of India Limited
In the period from 2004 through 2011, the Company entered into and amended certain license and supply agreements with Serum Institute. The original license agreement with Serum Institute was a collaborative Development and Manufacturing Arrangement (“DMA”) to develop agreed upon potential commercial product candidates using the Company’s PolyXen™ technology. Serum Institute then endeavored to further develop the potential commercial product candidates and eventually initiate pre-clinical and clinical trials at their own cost. The agreement was amended in 2011, resulting in the surrender of development rights for 14 potential commercial product candidates in 2012, which were vested to Serum Institute under the terms of the previous agreements, back to the Company.
Following the 2011 amendment, Serum Institute retained an exclusive license to use the Company’s PolyXen™ technology to research and develop one potential commercial product, Polysialylated Erythropoietin (“PSA-EPO”). Serum Institute will be responsible for conducting all pre-clinical and clinical trials required to achieve regulatory approvals within the certain predetermined territories at Serum Institute’s own expense. The royalty payment schedule based on net revenues on the future commercial sales of PSA-EPO under the DMA was also modified as a result of the 2011 amendment. Royalty payments are payable by Serum Institute to the Company for net sales to certain customers in the Serum Institute sales territory. Royalty payments are payable by the Company to Serum Institute for net sales received by the Company over the term of the license. There are no milestone or other research-related payments due under the DMA.
Through December 31, 2015, the Company and Serum Institute continued to engage in research and development activities with no resultant commercial products. No royalty revenue or expense was recognized by the Company related to the Serum Institute arrangement during the years ended December 31, 2015 and 2014.
Serum Institute is a related party of the Company, with a share ownership of approximately 8.5% and 9.2% of the total issued common stock of the Company as of December 31, 2015 and 2014, respectively. On December 31, 2014, the Company granted Serum Institute a warrant to purchase 96,970 shares of common stock in connection with ongoing collaborative activities. See Note 9, Stockholders’ Equity, for further information on the warrant.
OJSC Pharmsynthez
In November 2011, the Company entered into a collaborative research and development license agreement with OJSC Pharmsynthez (the “Pharmsynthez Arrangement”) pursuant to which the Company granted an exclusive license to Pharmsynthez to develop, commercialize and market six product candidates based on the Company’s PolyXen™ and ImuXen™ technology in certain territories. In exchange, Pharmsynthez granted an exclusive license to the Company to use any pre-clinical and clinical data developed by Pharmsynthez, within the scope of the Pharmsynthez Arrangement, and to engage in further research, development and commercialization of drug candidates outside of certain territories at the Company’s own expense.
In July 2015, the Company entered into the SPA with Pharmsynthez providing for the issuance of certain promissory notes and certain warrants to purchase shares of the Company’s common stock. See Note 8, Hybrid Debt Instrument, for discussion of the SPA and Note 11, Stockholders’ Equity, for discussion of the warrants.
In November 2015, the Company entered into the APA with the Sellers. Pursuant to the APA, Kevelt will transfer to the Company certain intellectual property rights with respect to the immunomodulatory product candidate Virexxa® held by Kevelt. See Note 3, Acquisitions, for further discussion of the APA. The APA also provides for the Company’s issuance of certain promissory notes and certain warrants to purchase shares of the Company’s common stock. See Note 11, Stockholders’ Equity, for discussion of the warrants.
Pharmsynthez is a related party of SynBio, which is an affiliate of the Company. In addition, one of the Company’s directors is also a director of SynBio and Pharmsynthez.
| F-29 |
| 5. | Property and Equipment, net |
Property and equipment, net consists of the following:
| December 31, 2015 | December 31, 2014 | |||||||
| Laboratory equipment | $ | 249,969 | $ | 254,150 | ||||
| Office and computer equipment | 35,190 | 189,459 | ||||||
| Leasehold improvements | 26,841 | 92,354 | ||||||
| Furniture and fixtures | 20,263 | 50,150 | ||||||
| Property and equipment | 332,263 | 586,113 | ||||||
| Less accumulated depreciation | (270,242 | ) | (466,664 | ) | ||||
| Property and equipment – net | $ | 62,021 | $ | 119,449 | ||||
In connection with the closing of the London office in March 2015, the Company disposed of approximately $247,000 of depreciated fixed assets with a net book value of approximately $6,000 for cash proceeds of approximately $8,000, resulting in a gain of approximately $2,000. Depreciation expense was $48,750 and $83,863 for the years ended December 31, 2015 and 2014, respectively.
| 6. | Goodwill and Indefinite-Lived Intangible Assets |
Goodwill
A reconciliation of the change in the carrying value of goodwill is as follows:
| Balance as of January 1, 2014 | $ | 3,665,199 | ||
| Acquired from acquisitions | 4,129,248 | |||
| Disposed with Hive Out Agreement | (4,129,248 | ) | ||
| Foreign currency translation | (200,042 | ) | ||
| Balance as of December 31, 2014 | $ | 3,465,157 | ||
| Foreign currency translation | (181,778 | ) | ||
| Balance as of December 31, 2015 | $ | 3,283,379 |
The goodwill acquired from the Acquisition was disposed in connection with the Hive Out Agreement. See Footnote 3, Acquisitions, for further discussion on the Acquisition and the Hive Out Agreement. As of October 1, 2015 and 2014, the dates of the Company’s annual impairment review, the fair value of the Company’s goodwill balance significantly exceeded its carrying value.
Indefinite-Lived Intangible Assets
The Company’s acquired indefinite-lived intangible asset, OncoHist™, is IPR&D relating to the Company’s business combination with SymbioTec in 2012. The carrying value of OncoHist™ was $9.24 million and $9.75 million as of December 31, 2015 and 2014, respectively. No impairment was recorded during the years ended December 31, 2015 and 2014. The changes in the carrying value reflected herein are solely comprised of the effects of changes in foreign currency.
OncoHist™ is not yet commercialized and has not yet begun to be amortized as of December 31, 2015.
| F-30 |
| 7. | Accrued Expenses |
Accrued expenses consist of the following:
| December 31, 2015 | December 31, 2014 | |||||||
| Accrued payroll and benefits | $ | 625,289 | $ | 67,120 | ||||
| Accrued professional fees | 413,945 | 574,186 | ||||||
| Accrued research costs | 145,026 | 573,879 | ||||||
| Accrued interest | 77,857 | – | ||||||
| Other | 224,929 | 194,506 | ||||||
| $ | 1,487,046 | $ | 1,409,691 | |||||
| 8. | Hybrid Debt Instrument |
Securities Purchase Agreement
On July 1, 2015, the Company entered into the SPA with Pharmsynthez providing for the issuance of a minimum of a $3 million 10% Senior Secured Collateralized Convertible Promissory Note (the “SPA Note”). The SPA also provides for the issuance of certain warrants up to the amount of the SPA Note. See Note 11, Stockholders’ Equity, for discussion of the accounting treatment of the warrants. The convertible debt and its embedded debt-like features have been recorded on the face of the consolidated balance sheet within current liabilities as an aggregate hybrid debt instrument with a balance of $3.7 million as of December 31, 2015.
On July 1, 2015, the Company issued the SPA Note for $3 million plus a warrant to purchase 10 million shares of common stock in accordance with the terms of the SPA. In the event the SPA Note remains outstanding at April 1, 2016, Pharmsynthez will be granted an additional warrant to purchase $10 million shares of common stock. The SPA Note carries a term of one year and is convertible, in whole or in part, at the option of Pharmsynthez into shares of common stock at a conversion price of $4.95. The SPA Note bears interest at the rate of 10% annually, payable quarterly in cash or, at the Company’s option, in shares of common stock at the lessor of $4.95 or the then applicable conversion price. At any point after six months following the issuance of the SPA Note, but before the maturity date of the SPA Note, the Company has the option of prepayment of the SPA Note and any accrued interest. If the Company exercises the prepayment option, the Company is obligated to pay the outstanding principal amount of the SPA Note and accrued interest multiplied by 115%. If the SPA Note is converted or redeemed, prior to the maturity date, the Company will pay cash to Pharmsynthez equal to the interest that would have accrued from the conversion or redemption date to the maturity date.
Upon a qualifying capital raise in which the Company obtains financing of $7 million or greater (“Capital Raise”), Pharmsynthez has the option to either redeem the SPA Note for cash at the balance of principal plus accrued interest multiplied by 115% or convert the principal plus accrued interest multiplied by 115% into common stock at the effective conversion price. In the event of default, as defined under the terms of the SPA Note, all obligations will be immediately due and payable and the interest rate will increase to 18% annually. The Company determined these two features represent contingent put options for Pharmsynthez.
The Company concluded the two contingent put option features related to a Capital Raise and event of default, the SPA Note conversion feature and the ability for interest to be paid in shares of common stock feature each meet the definition of a derivative under ASC Topic 815, Derivatives and Hedging (“ASC 815”), and require bifurcation and accounting as embedded derivatives. The four embedded derivatives, which were bifurcated and individually fair valued by the Company, have been recorded as a compound derivative within the Hybrid Debt Instrument. The Company calculated the fair values of each individual embedded derivative by taking the difference between the fair value of the SPA Note with each embedded derivative and the fair value of the SPA Note without the individual embedded derivative. The Company calculated the fair values using the discounted present value of each embedded derivative value as determined by Monte Carlo Simulations. The key valuation assumptions used consist of the Company’s stock price, the risk free interest rate and expected volatility. The embedded derivatives were recorded within the Hybrid Debt Instrument as a compound derivative liability at an estimated fair value of $1.4 million at issuance and created an offsetting debt discount on the consolidated balance sheet that will be amortized over the life of the SPA Note using the effective interest rate method.
The fair value of the compound derivative is remeasured at each report date until settled, with changes in fair value recognized in the consolidated statement of comprehensive loss as a gain or loss on derivative. The fair value of the compound derivative increased $2.1 million since issuance to $3.5 million as of December 31, 2015. This change was recognized as a loss in Other Expense in the consolidated statement of comprehensive loss for the year ended December 31, 2015.
| F-31 |
The key assumptions used to calculate the estimated fair value of the compound derivative liability at issuance and as of December 31, 2015 are as follows:
| December 31, 2015 | July 1, 2015 | |||||||
| Company stock price | $ | 16.83 | $ | 7.26 | ||||
| Expected volatility (%) | 105 | % | 115 | % | ||||
| Risk-free interest rate (%) | 0.65 | % | 0.28 | % | ||||
The offset to debt arising from the recording of the compound derivative liability, the warrants and the associated issuance costs exceeded the debt proceeds by approximately $60,000. This amount was recorded as a loss in Other Expense in the consolidated statement of comprehensive loss for the year ended December 31, 2015.
Interest expense related to the SPA Note of approximately $154,000 was recognized in Interest Expense in the consolidated statement of comprehensive loss for the year ended December 31, 2015. Of this amount, approximately $78,000 is recorded as accrued interest on the hybrid debt instrument and approximately $76,000 was settled in shares of issuable common stock as of December 31, 2015, as provided in the APA.
The Company also evaluated the provision in the SPA Note that increases the annual interest rate in the event of default and concluded that the initial value of this contingent feature is immaterial to the consolidated financial statements as of December 31, 2015. The Company will evaluate the value of this contingent feature at each reporting period.
Asset Purchase Agreement
In November 2015, the Company entered into the APA with Kevelt and Pharmsynthez providing for the issuance of 10% Senior Secured Convertible Promissory Notes of up to $3.5 million to Pharmsynthez (the “APA Notes”) and warrants to purchase a number of shares of the Company’s common stock equal to 50% of the number of shares issuable under the APA Notes. In the event that the APA Notes remain outstanding at May 11, 2016, Pharmsynthez shall be granted an additional warrant to purchase an additional number of shares of the Company’s common stock equal to 50% of the number of shares issuable under the APA Notes. The APA Notes will be issued under similar terms and conditions as the SPA Note. No APA Notes were issued by the Company during the year ended December 31, 2015.
| 9. | Fair Value Measurements |
ASC Topic 820, Fair Value Measurement, defines fair value as the price that would be received to sell an asset or be paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Company applies the following fair value hierarchy, which prioritizes the inputs used to measure fair value into three levels and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement. Level 1 inputs are quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. Level 2 utilizes quoted market prices in markets that are not active, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency. Level 3 inputs are unobservable inputs for the asset or liability in which there is little, if any, market activity for the asset or liability at the measurement date.
The Company’s cash and restricted cash are measured at fair value and are classified as Level 1 in the fair value hierarchy. The carrying amount of certain of the Company’s financial instruments approximate fair value due to their short maturities. The Company’s derivative liabilities are measured at fair value on a recurring basis and are classified as Level 3 in the fair value hierarchy.
The following table provides a summary of the changes in fair value of the compound derivative measured at fair value on a recurring basis using significant unobservable inputs during the year ended December 31, 2015.
| Balance as of January 1, 2015 | $ | – | ||
| Issuance of compound derivative instrument | (1,419,105 | ) | ||
| Change in fair value of compound derivative instrument | (2,125,117 | ) | ||
| Balance as of December 31, 2015 | $ | (3,544,222 | ) |
There were no financial instruments classified as Level 3 in the fair value hierarchy during the year ended December 31, 2014.
| F-32 |
| 10. | Income Taxes |
The Company accounts for income taxes using the liability method under ASC Topic 740, Income Taxes. Under this method, deferred tax assets and liabilities are determined based on temporary differences resulting from the different treatment of items for tax and financial reporting purposes. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to reverse. Additionally, the Company must assess the likelihood that deferred tax assets will be recovered as deductions from future taxable income. The Company has provided a full valuation allowance on the Company’s deferred tax assets because the Company believes it is more likely than not that its deferred tax assets will not be realized. The Company evaluates the recoverability of its deferred tax assets on a quarterly basis. Currently, there is no provision for income taxes as the Company has incurred losses to date.
The components of (loss) before income taxes are as follows:
| Year ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Domestic (U.S.) | $ | (7,724,418 | ) | $ | (4,040,654 | ) | ||
| Foreign (U.K.) | (4,767,363 | ) | (10,003,427 | ) | ||||
| Foreign (Germany) | (15,690 | ) | (263,023 | ) | ||||
| Loss before income taxes | $ | (12,507,471 | ) | $ | (14,307,104 | ) | ||
The reconciliation of income tax provision (benefit) at the U.S. corporation tax rate, being the rate applicable to the country of domicile of Xenetic Biosciences, Inc. to net income tax provision (benefit) is as follows:
| Year ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Federal | $ | (4,252,540 | ) | $ | (4,860,256 | ) | ||
| State | (276,601 | ) | (145,209 | ) | ||||
| Increase in tax losses not recognized | 2,238,879 | 4,949,805 | ||||||
| Permanent differences, net | 800,891 | (1,529,190 | ) | |||||
| Mark to market | 722,540 | – | ||||||
| Foreign rate differential | 502,357 | 1,184,770 | ||||||
| Share-based payments, net | 308,888 | 505,035 | ||||||
| Other | – | 7,273 | ||||||
| Enhanced research and development tax credits | (44,414 | ) | (112,228 | ) | ||||
| Net provision (benefit) for income taxes | $ | – | $ | – | ||||
Deferred tax assets and liabilities reflect the net tax effect of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets are as follows:
| Year ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Deferred tax assets: | ||||||||
| U.K. net operating loss carryforwards | $ | 9,402,398 | $ | 9,198,798 | ||||
| U.K. capital loss carryforwards | 1,775,932 | 1,874,254 | ||||||
| U.S. federal net operating loss carryforwards | 1,659,050 | 923,816 | ||||||
| Share-based payments | 1,313,226 | 52,320 | ||||||
| Enhanced research and development tax credits | 852,272 | 786,342 | ||||||
| Germany net operating loss carryforwards | 401,906 | 393,638 | ||||||
| U.S. state net operating loss carryforwards | 422,622 | 233,825 | ||||||
| Accrued expenses | 44,557 | 157,329 | ||||||
| Depreciation | 25,823 | 37,703 | ||||||
| Other | 4,998 | 115,384 | ||||||
| Total deferred tax assets before valuation allowance | 15,902,784 | 13,773,409 | ||||||
| Deferred tax liabilities: | ||||||||
| Indefinite-lived intangible asset | (2,918,518 | ) | (3,080,096 | ) | ||||
| Debt discount | (578,346 | ) | – | |||||
| Total deferred tax liabilities | (3,496,864 | ) | (3,080,096 | ) | ||||
| Less valuation allowance | (15,324,438 | ) | (13,773,409 | ) | ||||
| Total net deferred tax liability | $ | (2,918,518 | ) | $ | (3,080,096 | ) | ||
| F-33 |
For the years ended December 31, 2015 and 2014, the Company had U.K. net operating loss carryforwards of $47.01 million and $45.99 million, respectively, U.S. federal net operating loss carryforwards of $5.30 million and $2.95 million, respectively, U.S. state net operating loss carryforwards of $5.28 million and $2.92 million, respectively, and Germany net operating loss carryforwards of approximately $1.27 million and $1.25 million, respectively. The U.K. and Germany net operating loss carryforwards can be carried forward indefinitely. The U.S. federal and state net operating loss carryforwards begin to expire in 2032.
The Company’s ability to use its operating loss carryforwards and tax credits generated in the U.S. to offset future taxable income is subject to restrictions under Section 382 of the United States Internal Revenue Code (the “Internal Revenue Code”). These restrictions may limit the future use of the operating loss carryforwards and tax credits if certain ownership changes described in the Internal Revenue Code occur. Future changes in stock ownership may occur that would create further limitations on the Company’s use of the operating loss carryforwards and tax credits. In such a situation, the Company may be required to pay income taxes, even though significant operating loss carryforwards and tax credits exist.
The Company’s ability to use its operating loss carryforwards and tax credits generated in the U.K. are subject to restrictions under U.K. tax legislation. These regulations may limit the future use of operating loss carryforwards if there is a change in ownership and a change in the nature or conduct of the business carried on by the Company, and in certain circumstances where there is a change in the nature or conduct of the business only. In such cases the carryforwards would cease to be available to set against future income.
The Company’s ability to use its operating loss carryforwards and tax credits generated in Germany are also subject to restrictions under German tax legislation. These regulations may limit the future use of operating loss carryforwards if there is a change in ownership. In such cases the carryforwards would cease to be available to set against future income.
As of December 31, 2015 and 2014, the Company did not record any uncertain tax positions. As of January 1, 2014, the Company had recorded an uncertain tax position due to a claim for research and development tax credits with a full valuation allowance. During 2014, the Company determined that it is unable to obtain and compile the necessary information to support and defend the recoverability of the research and development tax credits, resulting in the write-off of the previously fully reserved balance. The changes to uncertain tax positions for 2015 and 2014 were as follows:
| Year ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Uncertain tax benefits as of January 1 | $ | – | $ | 185,961 | ||||
| Gross adjustments in tax positions | – | (185,961 | ) | |||||
| Uncertain tax positions as of December 31 | $ | – | $ | – | ||||
The Company files income tax returns in the U.S. federal tax jurisdiction and Massachusetts state tax jurisdiction, and certain foreign tax jurisdictions. The Company is subject to examination by the U.S. federal, state, foreign, and local income tax authorities for calendar tax years ending 2012 through 2015 due to available net operating loss carryforwards and research and development tax credits arising in those years. The Company has not been notified of any examinations by the Internal Revenue Service or any other tax authorities as of December 31, 2015. The Company has not recorded any interest or penalties for unrecognized tax benefits since its inception.
Potential 382 Limitation
The Company’s net operating loss and tax credit carryforwards are subject to review and possible adjustment by the Internal Revenue Service. The Company’s ability to utilize our net operating loss (“NOL”) and alternative minimum tax (“AMT”) and research and development credit (“R&D”) carryforwards may be substantially limited due to ownership changes that may have occurred or that could occur in the future, as required by Section 382 of the Internal Revenue Code of 1986, as amended (the “Code”), as well as similar state provisions. These ownership changes may limit the amount of NOL, AMT and R&D credit carryforwards that can be utilized annually to offset future taxable income and tax, respectively. In general, an ownership change, as defined in Section 382 of the Code, results from a transaction or series of transactions over a three-year period resulting in an ownership change of more than 50% of the outstanding stock of a company by certain stockholders or public groups.
| F-34 |
The Company has not completed a study to assess whether one or more ownership changes have occurred since we became a loss corporation as defined in Section 382 of the Code, but we believe that it is likely that an ownership change has occurred. If we have experienced an ownership change, utilization of the NOL, AMT and R&D credit carryforwards would be subject to an annual limitation, which is determined by first multiplying the value of our common stock at the time of the ownership change by the applicable long-term, tax-exempt rate, and then could be subject to additional adjustments, as required. Any such limitation may result in the expiration of a portion of the NOL, AMT or R&D credit carryforwards before utilization. Until a study is completed and any limitation known, no amounts are being considered as an uncertain tax position or disclosed as an unrecognized tax benefit under ASC 740. Any carryforwards that expire prior to utilization as a result of such limitations will be removed from deferred tax assets with a corresponding adjustment to the valuation allowance. Due to the existence of the valuation allowance, it is not expected that any potential limitation will have a material impact on our operating results.
From time to time the Company may be assessed interest or penalties by major tax jurisdictions, namely the state of Massachusetts. As of December 31, 2015, the Company had no material unrecognized tax benefits and no adjustments to liabilities or operations were required. No interest and penalties have been recognized by the Company to date.
The Company net operating loss carryforwards are subject to review and possible adjustment by the Internal Revenue Service and are subject to certain limitations in the event of cumulative changes in the ownership interest of significant stockholders over a three-year period in excess of 50%.
| 11. | Stockholders’ Equity |
Common Stock
Each share of common stock entitles the holder to one vote on all matters submitted to a vote of the Company’s stockholders. Common stockholders are entitled to dividends when and if declared by the Board of Directors. In the event of any voluntary or involuntary liquidation, dissolution or winding-up of the Company, the holders of common stock are entitled to share ratably in the assets of the Company available for distribution.
On September 30, 2015, the Company filed an Amendment to the Articles of Incorporation with the Secretary of State of the State of Nevada to increase the authorized shares of common stock of the Company and change the par value per share of common stock (the “Amendment”). The Amendment authorizes the Company to issue 45,454,546 shares of Common Stock and changes the par value to $0.001 per share. Prior periods have been reclassified to reflect the change in the par value per share to conform to the presentation in the present period.
On January 30, 2014, the Company announced the amendment of the licensing agreement with Baxter in which certain financial and timing aspects of the agreement were modified. As a result, the Company is entitled to receive certain amounts in development, regulatory and sales milestone payments as well as increased royalties on potential net sales. In addition, Baxter SA made a direct equity investment of $10 million in cash in exchange for 324,097 shares of the Company’s common stock. During June 2015, in connection with the separation of its biopharmaceuticals business to form Baxalta Incorporated (“Baxalta”), Baxter assigned all of its rights and obligations under its existing agreement with the Company to Baxalta.
| F-35 |
In December 2015, 333,334 shares of new common stock were issued to Dr. Genkin and Mr. Surkhov in connection with the APA. As a result, the Company recorded approximately $3.74 million, the fair value of the proportional consideration provided, as a prepayment within current assets on the consolidated balance sheet as of December 31, 2015.
Warrants
In connection with the Company’s collaboration and consultant agreements and financing arrangements, the Company issues warrants to purchase shares of common stock. These warrants were fair valued at issuance date using the Black-Scholes option pricing model. The warrants are subject to re-measurement at each reporting period until the measurement date is reached. Expense is recognized on a straight-line basis over the expected service period or at the date of issuance, if there is not a service period.
Warrants Related to Collaboration and Consulting Agreements
In 2010, the Company granted Baxter SA a warrant to purchase 139,040 new shares of common stock. During June 2015, in connection with the separation of its biopharmaceuticals business to form Baxalta, Baxter assigned the warrant to Baxalta. The warrant was exercisable immediately after issuance and had an initial expiration date of June 30, 2015, which the Company expects to extend subsequent to December 31, 2015. These warrants, which were fair valued at $932,000 at the time of issuance, were not exercised during the years ended December 31, 2015 and 2014.
In 2011, the Company granted SynBio a warrant to purchase 107,443 new shares of common stock, which was exercisable two years after issuance and expires on December 2, 2016 (“SynBio 2011 Warrant”). On December 31, 2014, SynBio was granted a warrant to purchase 204,394 new shares of common stock at an exercise price of $25.41 per share (“SynBio 2014 Warrant”). The SynBio 2014 Warrant is exercisable in four equal tranches, each with separate non-market, performance-based vesting criteria. The Company uses its judgment to assess the probability and timing of SynBio achieving this vesting criteria and estimated that it is not probable that the vesting criteria for any tranche will be achieved. As a result, the Company did not recognize expense related to this warrant during the years ended December 31, 2015 and 2014. These judgments are reassessed at each reporting period until the measurement date is reached. Upon issuance of the SynBio 2014 Warrant on December 31, 2014, the SynBio 2011 Warrant was canceled and of no further force and effect.
In connection with the SynBio 2014 Warrant grant, warrants to purchase 9,697 aggregate new shares of common stock were issued to SynBio and Pharmsynthez non-director designees (“SynBio Partner Warrants”) on December 31, 2014 under the same terms and conditions of the SynBio 2014 Warrant. The Company estimated that it is not probable that the vesting criteria for any trance will be achieved and, as a result, the Company did not recognize expense related to the SynBio Partner Warrants during the years ended December 31, 2015 and 2014. The SynBio 2014 Warrant and SynBio Partner Warrants expire on December 30, 2019 and no warrants were exercised during the years ended December 31, 2015 and 2014.
On December 31, 2014, the Company granted Serum Institute a warrant to purchase 96,970 new shares of common stock at an exercise price of $25.41 per share (“Serum Institute 2014 Warrant”). The Serum Institute 2014 Warrant, which was fair valued at approximately $480,000 at the time of issuance, is exercisable in two equal tranches, each with separate non-market, performance-based vesting criteria. The Company uses its judgment to assess the probability and timing of Serum Institute achieving this vesting criteria and estimated that it is probable that the vesting criteria will be achieved for each tranche. These judgments are reassessed at each reporting period until the measurement date is reached.
| F-36 |
In connection with the Serum Institute 2014 Warrant grant, warrants to purchase 4,849 aggregate new shares of common stock were issued to Serum Institute employees (“Serum Institute Partner Warrants”) on December 31, 2014 under the same terms and conditions of the Serum Institute 2014 Warrant. The Serum Institute Partner Warrants were fair valued at approximately $24,000 at the time of issuance. The Company recognized warrant expense of $706,500 and zero during the years ended December 31, 2015 and 2014, respectively, related to the Serum Institute 2014 Warrant and Serum Institute Partner Warrants. The Serum Institute 2014 Warrant and Serum Institute Partner Warrants expire on December 30, 2019 and no warrants were exercised during the years ended December 31, 2015 and 2014.
On December 31, 2014, the Company granted a nonemployee director a warrant to purchase 48,485 new shares of common stock at an exercise price of $25.41 per share for services provided to the Company. The warrant is a standalone instrument that is not puttable or mandatorily redeemable by the holder and is classified as an equity award. The Company determined that the fair value of the warrant is more reliably measureable than the fair value of the services received. As a result, the warrant was fair valued at approximately $240,000 at the time of issuance. As performance was completed and the measurement date reached at the time of issuance, the Company recorded expense of approximately $240,000 to general and administrative expenses in the consolidated statement of comprehensive loss during the year ended December 31, 2014. The warrant is exercisable two years after issuance and expires on December 30, 2019.
In August 2015, the Company issued a warrant to purchase approximately 25,243 shares of common stock to a consultant upon engagement of services to be provided to the Company. The warrant has a term of five years and an exercise price of $25.41. The warrant is a standalone instrument that is not puttable or mandatorily redeemable by the holder and is classified as an equity award. The Company determined that the fair value of the warrant is more reliably measureable than the fair value of the services received. As a result, the warrant was fair valued at approximately $227,000 at the time of issuance using the Black-Scholes option pricing model. As all services were completed as of December 31, 2015, the warrant expense was recognized during the year ended December 31, 2015.
Key assumptions used in the Black-Scholes option pricing model for warrants related to collaboration and consultant agreements granted during the years ended December 31, 2015 and 2014 are as follows:
| 2015 | 2014 | |||||||
| Weighted-average expected dividend yield (%) | – | – | ||||||
| Weighted-average expected volatility (%) | 104.81 | 103.32 | ||||||
| Weighted-average risk-free interest rate (%) | 1.03 | 0.96 | ||||||
| Weighted-average expected life of option (years) | 5.00 | 5.00 | ||||||
| Weighted-average exercise price ($) | 25.41 | 25.41 | ||||||
| Model used | Black-Scholes | Black-Scholes | ||||||
| F-37 |
Warrants Related to Financing Arrangements
In connection with the Company’s issuance of the SPA Note on July 1, 2015, the Company issued a warrant to purchase 303,031 shares of common stock in accordance with the terms of the SPA (the “Warrant”). The Warrant has a five-year term and is exercisable commencing January 1, 2016. The exercise price per share under the Warrant is the lessor of $6.60 or 120% of the Capital Raise price, in the event there is a Capital Raise. If the SPA Note is not repaid or converted on or before six months from the date of issuance, the Holder will be issued an additional warrant to purchase 303,031 shares of common stock under the same terms as the Warrant (the “Contingent Warrant”, or together referred to as the “Warrants”). The Company determined there is a high probability that the SPA Note will not be repaid or converted within the period six months from the date of issuance, resulting in the issuance of the Contingent Warrant. As such, the Company concluded the Contingent Warrant is considered to be issued and outstanding as of the SPA Note issuance date in accordance with ASC 815. The fair values of the Warrants were calculated using the Black-Scholes option pricing model. The key valuation assumptions used consist of the Company’s stock price, a risk free rate of 1.70% and an expected volatility of 125%. Using an allocation of the SPA Note proceeds between the relative fair values of the Warrants and the SPA Note, the Company recorded the Warrants at a value of $1.6 million on the consolidated balance sheet as equity paid-in-capital. This created a debt discount of $1.6 million that will amortize from the date of issuance through the term of the SPA Note.
In November 2015, the Company entered into the APA with Kevelt and Pharmsynthez, which provided for the issuance of certain warrants to purchase a number of share of the Company’s common stock equal to 50% of the number of shares issuable under the APA Notes. In the event that the APA Notes remain outstanding at May 11, 2016, Pharmsynthez shall be granted an additional warrant to purchase an additional number of shares of the Company’s common stock equal to 50% of the number of shares issuable under the APA Notes. The warrants will be issued under the same terms and conditions as the warrants under the SPA. No warrants under the APA were issued by the Company during the year ended December 31, 2015.
| 12. | Share-Based Payments |
Total share-based payments related to employee and nonemployee stock options, common stock awards and JSOP awards was $2,594,113 and $1,513,238 for the years ended December 31, 2015 and 2014, respectively.
Share-based payments is classified in the consolidated statements of comprehensive loss as follows:
| Year Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Research and development expenses | $ | 229,964 | $ | 952,829 | ||||
| General and administrative expenses | 2,364,149 | 560,409 | ||||||
| $ | 2,594,113 | $ | 1,513,238 | |||||
Stock Option Modifications
Prior to the Acquisition in 2014, the Company had two incentive stock plans, the Lipoxen plc Unapproved Share Option Plan (the “2000 Stock Plan”) and the Xenetic Biosciences plc 2007 Share Option Scheme (the “2007 Stock Plan”). Subsequent to the Acquisition, the 2000 and 2007 Stock Plans were converted to reflect the new shares issued by the Company under the Scheme of Arrangement related to the Acquisition. As part of the conversion, option holders under the 2000 and 2007 Stock Plan have the right to subscribe for a number of shares of common stock in the Company (the “Replacement Option Shares”) in exchange for the cancellation and surrender by the option holder of the original options granted by the 2000 and 2007 Stock Plans. The number of Replacement Option Shares is determined in the same manner in which the shareholders of Xenetic UK were given the right to acquire shares of common stock in the Company according to the Acquisition. The aggregate exercise price payable in U.S. dollars for Replacement Option Shares is the same as the aggregate exercise price in pounds sterling of the original options, using a foreign currency exchange rate for pounds sterling into U.S. dollars quoted by Barclays Bank plc at 12 noon Greenwich Mean Time (“GMT”) on January 23, 2014, the date of the Acquisition. The conversion of the options is treated as an option modification. The Company accounted for the option modification under ASC Topic 718, Compensation – Stock Compensation, and determined the option modification did not result in incremental stock compensation cost that is material to the Company’s results of operations during the year ended December 31, 2014.
| F-38 |
During the year ended December 31, 2015, the Company modified 303,031 employee stock option awards to extend the expiry dates through March 31, 2016. The Company accounted for the option modification under ASC Topic 718, Compensation – Stock Compensation, and as a result, recognized $25,008 in incremental compensation expense during the year ended December 31, 2015.
Stock Options
The Company grants stock option awards to employees and nonemployees with varying vesting terms under the Xenetic Biosciences, Inc. Equity Incentive Plan (“Stock Plan”). The Company measures the fair value of stock option awards using the Black-Scholes option pricing model, which uses the assumptions noted in the tables below, including the risk-free interest rate, expected term, share price volatility, dividend yield and forfeiture rate. The risk-free interest rate is based upon the U.S. Treasury yield curve in effect at the time of grant, with a term that approximates the expected life of the option. For employee stock options issued in 2015 and 2014 that qualify as “plain vanilla” stock options in accordance with Staff Accounting Bulletin No. 110 (“SAB 110”), the expected term is based on the simplified method, as defined by SAB 110. The Company has a limited history of stock option exercises, which does not provide a reasonable basis for the Company to estimate the expected term of employee stock options. For all other employee stock options, the Company estimates the expected life using judgment based on the anticipated research and development milestones of the Company’s clinical projects and behavior of the Company’s employees. The expected life of nonemployee options is the contractual life of the option. The Company determines the expected volatility based on a blended volatility rate of its own historical volatility with that of comparable publicly traded companies with product candidates in similar therapeutic areas and stages of nonclinical and clinical development to the Company’s product candidates. The Company has applied an expected dividend yield of 0% as the Company has not historically declared a dividend and does not anticipate declaring a dividend during the expected life of the options. Further, the Company has applied a forfeiture rate of 0% as the Company has not historically experienced forfeitures.
During the years ended December 31, 2015 and 2014, 493,945 and 32,731 total stock options to purchase shares of common stock were granted under the Stock Plan, respectively, with a weighted average grant date fair value per option share of $9.35 and $7.58, respectively. During the year ended December 31, 2014, 1,984,080 stock options were exercised and cash received from those stock option exercises was $101,933. No stock options were exercised during the year ended December 31, 2015.
During the year ended December 31, 2015 and 2014, 161,657 and 20,687 total stock options vested, with total fair values of $1,391,450 and $115,864, respectively. As of December 31, 2015, there was $2,931,117 of unrecognized share-based payments related to employee stock options that are expected to vest. The Company expects to recognize this expense over a weighted-average period of approximately 2 years.
Key assumptions used in the Black-Scholes option pricing model for options granted to employees during the years ending December 31, 2015 and 2014 are as follows:
| Year Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Weighted-average expected dividend yield (%) | – | – | ||||||
| Weighted-average expected volatility (%) | 124.17 | 103.36 | ||||||
| Weighted-average risk-free interest rate (%) | 0.44 | 1.48 | ||||||
| Weighted-average expected life of option (years) | 2.50 | 5.33 | ||||||
| Weighted-average exercise price ($) | 13.86 | 10.15 | ||||||
| Model used | Black-Scholes | Black-Scholes | ||||||
| F-39 |
The following is a summary of employee stock option activity for the years ended December 31, 2015 and 2014:
| Number of shares | Weighted-average exercise price | Weighted-average remaining life (years) | Aggregate intrinsic value | |||||||||||||
| Outstanding as of January 1, 2014 | 158,223 | 15.78 | ||||||||||||||
| Granted | 32,731 | 10.15 | ||||||||||||||
| Exercised | (60,125 | ) | 1.70 | $ | 509,622 | |||||||||||
| Expired | (3,967 | ) | 30.58 | |||||||||||||
| Outstanding as of December 31, 2014 | 126,862 | 20.53 | 6.86 | $ | 80,338 | |||||||||||
| Granted | 493,945 | 13.86 | ||||||||||||||
| Expired | (1,548 | ) | 15.51 | |||||||||||||
| Outstanding as of December 31, 2015 | 619,259 | 15.22 | 8.92 | $ | 1,915,942 | |||||||||||
| Vested or expected to vest as of December 31, 2015 | 619,259 | 15.22 | 8.92 | $ | 1,915,942 | |||||||||||
| Exercisable as of December 31, 2014 | 79,710 | $ | 19.71 | 5.48 | $ | 80,338 | ||||||||||
| Exercisable as of December 31, 2015 | 239,819 | $ | 17.17 | 7.78 | $ | 688,343 | ||||||||||
A summary of the status of the Company’s non-vested employee stock option shares as of December 31, 2015 and the changes during the year ended December 31, 2015 is as follows:
| Number of shares | Weighted-average grant date fair value | |||||||
| Balance as of January 1, 2015 | 47,152 | $ | 5.03 | |||||
| Granted | 493,945 | $ | 9.35 | |||||
| Vested | (161,657 | ) | $ | 8.61 | ||||
| Balance as of December 31, 2015 | 379,440 | $ | 9.14 | |||||
NonEmployee Stock Options
Share-based payments expense related to stock options granted to nonemployees is recognized as the services are rendered on a straight-line basis. The Company determined that the fair value of the stock options is more reliably measurable than the fair value of the services received. Compensation expense related to stock options granted to nonemployees is subject to re-measurement at each reporting period until the options vest.
During the years ended December 31, 2015 and 2014, 30,304 and 14,546 nonemployee stock options were granted under the Stock Plan, respectively, with a weighted average grant date fair value per option share of $13.13 and $7.75, respectively. No nonemployee stock options were exercised during years ended December 31, 2015 and 2014.
During the year ended December 31, 2015 and 2014, 17,857 and 7,756 total stock options vested, with total fair values of $195,575 and $62,121, respectively. As of December 31, 2015, there was $263,778 of unrecognized share-based payments related to nonemployee stock options that are expected to vest. The Company expects to recognize this expense over a weighted-average period of approximately 1.5 years.
Key assumptions used in the Black-Scholes option pricing model for nonemployees options during the years ended December 31, 2015 and 2014 are as follows:
| Year Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Weighted-average expected dividend yield (%) | – | – | ||||||
| Weighted-average expected volatility (%) | 120.51 | 116.22 | ||||||
| Weighted-average risk-free interest rate (%) | 1.54 | 1.62 | ||||||
| Weighted-average expected life of option (years) | 10.00 | 7.60 | ||||||
| Weighted-average exercise price ($) | 13.86 | 8.25 | ||||||
| Model used | Black-Scholes | Black-Scholes | ||||||
| F-40 |
The following is a summary of nonemployee stock option activity for the years ended December 31, 2015 and 2014:
| Number of shares | Weighted-average exercise price | Weighted-average remaining life (years) | Aggregate intrinsic value | |||||||||||||
| Outstanding as of January 1, 2014 | 12,592 | $ | 18.18 | 5.90 | $ | 49 | ||||||||||
| Granted | 14,546 | 8.25 | ||||||||||||||
| Outstanding as of December 31, 2014 | 27,138 | 12.86 | 7.60 | $ | 159 | |||||||||||
| Granted | 30,304 | 13.86 | ||||||||||||||
| Outstanding as of December 31, 2015 | 57,442 | 13.39 | 8.23 | $ | 220,764 | |||||||||||
| Vested or expected to vest as of December 31, 2015 | 57,442 | 13.39 | 8.23 | $ | 220,764 | |||||||||||
| Exercisable as of December 31, 2014 | 11,627 | $ | 13.73 | 6.40 | $ | 159 | ||||||||||
| Exercisable as of December 31, 2015 | 29,484 | $ | 13.37 | 7.37 | $ | 119,164 | ||||||||||
A summary of the status of the Company’s non-vested nonemployee stock option shares as of December 31, 2015 and the changes during the year ended December 31, 2015 is as follows:
| Number of shares | Weighted-average grant date fair value | |||||||
| Balance as of January 1, 2015 | 15,511 | $ | 8.16 | |||||
| Granted | 30,304 | $ | 13.13 | |||||
| Vested | (17,857 | ) | $ | 10.95 | ||||
| Balance as of December 31, 2015 | 27,958 | $ | 11.77 | |||||
Common Stock Awards
The Company granted common stock awards to several nonemployees in exchange for services provided. The Company measures the fair value of these awards using the fair value of the services provided or the fair value of the awards granted, whichever is more reliably measurable. The fair value measurement date of these awards is generally the date the performance of services is complete. The fair value of the awards is recognized as services are rendered on a straight-line basis.
A summary of the Company’s common stock awards granted and issued during the years ended December 31, 2015 and 2014 are as follows:
| Number of shares | ||||
| Balance as of January 1, 2014 | 13.943 | |||
| Granted | 104,006 | |||
| Issued | (98,327 | ) | ||
| Balance as of December 31, 2014 | 19,622 | |||
| Granted | 34,403 | |||
| Issued | (31,138 | ) | ||
| Balance as of December 31, 2015 | 22,887 | |||
The Company granted 34,403 and 5,679 shares of common stock during the years ended December 31, 2015 and 2014, respectively, in exchange for professional services. As all services were rendered in each respective period, expense related to common stock awards of $392,661 and $102,000 was recognized during the years ended December 31, 2015 and 2014, respectively.
| F-41 |
In December 2014, 98,327 shares of new common stock were granted and issued to FDS Pharma ASS (“FDS”) in consideration for the performance of services and termination of a prior collaboration agreement between Lipoxen and FDS. The Company determined that the fair value of the shares of common stock granted is more reliably measurable than the fair value of the services received. The Company assessed the fair value of one share of common stock on the measurement date to be $8.25. As performance by FDS was complete at the issuance date, the Company recorded expense of approximately $812,000 to research and development expense in the consolidated statement of comprehensive loss during the year ended December 31, 2014. FDS is a related party of SynBio, an affiliate of the Company.
Joint Share Ownership Plan
In 2010 and 2012, the Company issued 51,573 and 272,312 JSOP awards, respectively, to two senior executives under the JSOP. Under the JSOP, shares in the Company are jointly purchased at fair market value by the participating executives and the trustees of the JSOP trust, with such shares held in the JSOP trust. For US GAAP purposes the awards were valued as employee options and recorded as a reduction in equity as treasury shares until such time as they are exercised by the employee.
During 2011, the 2010 JSOP awards fully vested under the terms of the JSOP due to a significant change in beneficial ownership of the Company and the related compensation charges were fully recorded during periods prior to 2013 related to this accelerated vesting. During the first quarter of 2014, the 2012 JSOP awards fully vested under the terms of the JSOP due the achievement of specific share price hurdles and the related compensation charges were fully recorded during the first quarter of 2014 related to this accelerated vesting. As of December 31, 2014, all JSOP awards were fully vested. The Company recognized zero and $344,905, respectively, of JSOP compensation expense during the years ended December 31, 2015 and 2014. As of December 2015 and 2014, there were 323,885 JSOP awards issued.
| 13. | Employee Benefit Plans |
The Company has a defined contribution 401(k) savings plan (the “401(k) Plan”). The 401(k) Plan covers substantially all U.S. employees, and allows participants to defer a portion of their annual compensation on a pre-tax basis. Company contributions to the 401(k) Plan may be made at the discretion of the Board of Directors. During the year ended December 31, 2015 and 2014, the Company made contributions of approximately $34,000 and $32,000, respectively, to the 401(k) Plan.
In the U.K., the Company has adopted a defined contribution plan (the “UK Plan”) which qualifies under the rules established by HM Revenue & Customs. The UK Plan generally allows all U.K. employees to contribute a minimum of 3% of salary with no maximum limit. The Company contributes to the plan between 8% and 12% of the employee’s salary, depending upon seniority of the employee. The Company, at its discretion, may also contribute to an employee’s personal pension plan. The Company paid total contributions of approximately $144,000 and $108,000 during the years ended December 31, 2015 and 2014, respectively.
| 14. | Commitments and Contingent Liabilities |
Lease
In August 2013, the Company entered into an agreement to lease office and laboratory space in Lexington, Massachusetts under an operating lease with a commencement date of January 1, 2014 and a termination date of January 31, 2019. With the execution of this lease, the Company is required to maintain a $66,000 letter of credit as a security deposit, which is classified as a current asset within the consolidated balance sheet. In connection with the Lexington lease, the Company recorded $90,838 as prepaid rent as of December 31, 2015, with $61,377 recorded as a non-current asset. The Company also incurred a liability of $89,074 with respect to the Company’s contribution to the landlord’s leasehold improvements, of which $56,538 is outstanding as of December 31, 2015, with $38,791 recorded as a non-current liability. This liability is repayable as additional rent expense over the term of the lease and bears interest at 6%. In addition, the Company leased office space in London, U.K. during 2014 and 2015. The U.K. lease was terminated in March 2015 in accordance with the terms of the lease.
| F-42 |
The Company’s contractual commitments under all non-cancelable operating leases as of December 31, 2015 are as follows:
| As of December 31, | Total Operating Leases | |||
| 2016 | $ | 98,645 | ||
| 2017 | 102,604 | |||
| 2018 | 106,563 | |||
| 2019 | 8,908 | |||
| Total minimum lease payments | $ | 316,720 | ||
Rent expense is calculated on a straight-line basis over the term of the lease. Rent expense under the Company’s operating leases was $134,875 and $172,821 for the years ended December 31, 2015 and 2014, respectively.
Employment Agreements
The Company has contingent bonus compensation agreements with certain of the Company’s employees. The bonuses become payable upon the achievement of certain capital raise and stock listing metrics. The amount of contingent bonuses that may be paid out in future periods is a range of approximately $380,000 to $680,000 as of December 31, 2015.
| 15. | Related Party Transactions |
In May 2011, the Company received a short term unsecured loan facility of up to $1.7 million from SynBio, an affiliate of the Company, of which $395,000 was outstanding as of December 31, 2015 and 2014, respectively. A payment of $286,124 on the outstanding loan was made to SynBio during the year ended December 31, 2014. No payments were made during the year ended December 31, 2015. The loan had an interest rate of 8.04% per annum as of the date of grant, with interest payable upon repayment of the loan, which was to be seven months after the closing date of the loan. During 2012, the loan matured and it was agreed by both parties that the loan can be called due with full repayment of the outstanding principal including accrued interest upon future agreement by both parties. It was also agreed as of July 1, 2012 that no further interest on the outstanding loan balance would be accrued. The loan is recorded in “Loans due to related parties” within current liabilities as of December 31, 2015 and 2014. The loan does not bear interest at the prevailing market rate for instruments with similar characteristics.
During the years ended December 31, 2015 and 2014, the Company received research and consulting services from a nonemployee director of the Company. The total amount of services received was $72,594 and $74,582 for the years ended December 31, 2015 and 2014, respectively, with $17,791 and zero included in accounts payable on the consolidated balance sheet as of December 31, 2015 and 2014, respectively.
During the years ended December 31, 2015 and 2014, the Company also received consulting services from a firm owned by a nonemployee director of the Company. The total amount of services received was $4,000 and $133,381 for the years ended December 31, 2015 and 2014, respectively, with zero and $51,708 included in accounts payable on the consolidated balance sheet as of December 31, 2015 and 2014, respectively.
Please refer to Note 4, Significant Strategic Drug Development Collaborations, and Note 11, Stockholder’s Equity, for details on arrangements with collaboration partners and nonemployee directors that are also related parties.
| 16. | Subsequent Events |
The Company performed a review of events subsequent to the balance sheet date through the date the financial statements were issued and determined, except as disclosed herein, that there were no other such events requiring recognition or disclosure in the financial statements.
During the first quarter of 2016, the Company received total proceeds of $3.5 million in connection with the APA financing arrangement. The APA provided for the issuance of certain warrants to purchase a number of share of the Company’s common stock equal to 50% of the number of shares issuable under the APA Notes. The Warrant has a five-year term and is exercisable commencing March 31, 2016. The exercise price per share under the Warrant is the lessor of $6.60 or 120% of the Capital Raise price, in the event there is a Capital Raise. If the APA Note is not repaid or converted on or before six months from the date of issuance, the Holder will be issued an additional warrant under the same terms as the Warrant.
On June 1, 2016, the Company effected a reduction, on a 1 for 33 basis, in the authorized common stock, par value $0.001, along with a corresponding and proportional decrease in the number of shares issued and outstanding. See Note 2.
On August 26 and September 9, 2016 we issued convertible promissory notes in the amount of $178,000 and $322,000, respectively, to Pharmsynthez. The notes are convertible into shares of our common stock at any time at a conversion price of $4.00 per share (subject to price protection and usual and customary adjustments) or may be applied toward the Offering, at the option of Pharmsynthez.
On September 23, 2016, SynBio, LLC exchanged 970,000 shares of common stock in the Company for an equal number of shares of Series A Preferred Stock.
| F-43 |
XENETIC BIOSCIENCES, INC.
SCHEDULE II
VALUATION AND QUALIFYING ACCOUNTS
FOR THE YEARS ENDED DECEMBER 31, 2015 AND 2014
| Valuation Allowance on Deferred Tax Assets | Balance Beginning of Period | Additions (Deductions) Charged to (from) Income Tax Expense | Other Changes to Valuation Allowance |
Balance End of Period | ||||
| 2015 | $ | (13,773,409) | (1,551,029) | - | $ | (15,324,438) | ||
| 2014 | $ | (9,521,260) | (4,252,149) | - | $ | (13,773,409) |
| F-44 |
500,000 units consisting of One Share of Convertible Series B Preferred Stock and
a Class A Warrant to Purchase One Share of Common Stock and
2,000,000 units consisting of One Share of Convertible Series B Preferred Stock and
a Class B Warrant to Purchase One Share of Common Stock

______________________
PROSPECTUS
______________________
LADENBURG THALMANN
Through and including , 2016 (the 25th day after the date of this offering), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
______________________
PART II
Information Not Required in Prospectus
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the fees and expenses, other than underwriting discounts and commissions, payable in connection with the registration of the common stock hereunder. All amounts are estimates except the SEC registration fee and the FINRA filing fee.
| SEC registration fee | $ | 2,014 | ||
| FINRA Filing Fee | 5,900 | |||
| The NASDAQ Capital Market Listing Fee | 55,000 | |||
| Printing and Engraving Expenses | 10,000 | |||
| Legal Fees and Expenses | 480,000 | |||
| Accounting fees and expenses | 200,000 | |||
| Transfer Agent and Registrar Fees and Expenses | 1,500 | |||
| Miscellaneous | 10,000 | |||
| Total: | $ | 764,414 |
Item 14. Indemnification of Directors and Officers
Our officers and directors are indemnified as provided by the Nevada Revised Statutes and our bylaws.
Under the governing Nevada statutes, director immunity from liability to a company or its shareholders for monetary liabilities applies automatically unless it is specifically limited by a company's articles of incorporation. Our articles of incorporation do not contain any limiting language regarding director immunity from liability. Excepted from this immunity are:
| · | a willful failure to deal fairly with the company or its shareholders in connection with a matter in which the director has a material conflict of interest; |
| · | a violation of criminal law (unless the director had reasonable cause to believe that his or her conduct was lawful or no reasonable cause to believe that his or her conduct was unlawful); |
| · | a transaction from which the director derived an improper personal profit; and |
| · | willful misconduct. |
Our bylaws provide that we will indemnify our directors and officers to the fullest extent not prohibited by Nevada law; provided, however, that we may modify the extent of such indemnification by individual contracts with our directors and officers; and, provided, further, that we shall not be required to indemnify any director or officer in connection with any proceeding (or part thereof) initiated by such person unless:
| · | such indemnification is expressly required to be made by law; |
| · | the proceeding was authorized by our Board of Directors; |
| · | such indemnification is provided by us, in our sole discretion, pursuant to the powers vested us under Nevada law; or; |
| · | such indemnification is required to be made pursuant to the bylaws. |
Our bylaws provide that we will advance to any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he is or was a director or officer, of the company, or is or was serving at the request of the company as a director or executive officer of another company, partnership, joint venture, trust or other enterprise, prior to the final disposition of the proceeding, promptly following request therefore, all expenses incurred by any director or officer in connection with such proceeding upon receipt of an undertaking by or on behalf of such person to repay said amounts if it should be determined ultimately that such person is not entitled to be indemnified under our bylaws or otherwise.
| II-1 |
Our bylaws provide that no advance shall be made by us to an officer of the company, except by reason of the fact that such officer is or was a director of the company in which event this paragraph shall not apply, in any action, suit or proceeding, whether civil, criminal, administrative or investigative, if a determination is reasonably and promptly made: (a) by the board of directors by a majority vote of a quorum consisting of directors who were not parties to the proceeding, or (b) if such quorum is not obtainable, or, even if obtainable, a quorum of disinterested directors so directs, by independent legal counsel in a written opinion, that the facts known to the decision-making party at the time such determination is made demonstrate clearly and convincingly that such person acted in bad faith or in a manner that such person did not believe to be in or not opposed to the best interests of the company.
The underwriting agreement filed as Exhibit 1.1 to this registration statement provides for indemnification of us and our directors and officers by the underwriters against certain liabilities under the Securities Act and the Exchange Act.
Item 15. Recent Sales of Unregistered Securities
The following list sets forth information as to all securities we have sold since January 1, 2013, which were not registered under the Securities Act.
| 1. | On January 29, 2014 we entered into a stock purchase agreement (Purchase Agreement) with Baxalta (initially entered into with Baxter SA, subsequently transferred to Baxalta), pursuant to which we sold to Baxalta 324,097 shares of our common stock, par value $0.001 per share, (Shares) for $10 million (Purchase Price) at a price of $30.86 per share yielding a market cap of approximately $140 million. During 2015, Baxter agreed in writing to a further lock-up period expiring in June 2016 and certain other related restrictions. In connection with the separation of its biopharmaceuticals business to form Baxalta, Baxter assigned the Shares to Baxalta in 2015. |
| 2. | On December 31, 2014, in consideration of the assignment of certain intellectual property rights by Dmitry Genkin and FDS Pharma ASS to our subsidiary, Lipoxen Technologies Limited (Lipoxen), we issued to FDS Pharma ASS 98,327 shares of our common stock. FDS Pharma ASS is a related party of SynBio, LLC (SynBio) which is an affiliate of ours. |
| 3. | On December 31, 2014, we issued a warrant to purchase 204,394 shares of our common stock to SynBio in furtherance of our co-development clinical objectives. The initial exercise price for the purchase of the warrant is $25.41 per share with a term of five years from the grant date. Simultaneously, warrants to purchase 9,697 shares of our common stock were issued to SynBio and PJSC Pharmsynthez (Pharmsynthez) non-director designees under the same terms and conditions of the SynBio warrant. These warrants contain vesting triggers based on the achievement by SynBio of specific clinical development objectives. |
| 4. | On December 31, 2014, we issued a warrant to purchase 96,970 shares of our common stock, to Serum Institute of India Limited (Serum Institute) in furtherance of our co-development clinical objectives. The initial exercise price for the purchase of the warrant is $7.92 per share with a term of five years from the grant date. Simultaneously, warrants to purchase 4,852 shares of common stock were issued to Serum Institute non-director designees under the same terms and conditions of the Serum Institute warrant. These warrants contain vesting triggers based on the achievement by Serum Institute of specific clinical development objectives. Serum Institute is a related party of ours. | |
| 5. | On December 31, 2014, we issued a warrant to purchase 48,485 shares of our common stock to a nonemployee director for services provided to us. The initial exercise price for the purchase of the warrant is $7.92 per share with a term of five years from the grant date. This warrant was fully vested on the date of grant. |
| 6. | In August 2015, we issued 15,986 shares of our common stock to nonemployee consultants in exchange for services provided to the Company. We recorded $196,341 as the aggregate amount of consideration received for the associated services. |
| II-2 |
| 7. | In November 2015, in consideration of the assignment of certain intellectual property rights by Dr. Dmitry Genkin and Kirill Surkhov (together, Assignors) to Lipoxen Technologies Ltd., we issued 333,334 shares of our common stock to the Assignors pursuant to the terms of the Kevelt APA. We recorded $3.74 million as the aggregate amount of consideration received for these certain intellectual property rights. |
| 8. | In December 2015, we issued 15,152 shares of our common stock to nonemployee consultants in exchange for services provided to us. We recorded $221,00 as the aggregate amount of consideration for the associated services.
| |
| 9. | On April 28, 2016, we issued in the aggregate 4,418,491 new shares of our common stock to Pharmsynthez in connection with the conversion of certain convertible promissory notes issued in 2015 and 2016, as well as the closing of that certain Asset Purchase Agreement, dated November 13, 2015, (Kevelt APA) by and among the Company, our subsidiary, Lipoxen Technologies, LTD, Kevelt, an Estonian company (Kevelt) and Pharmsynthez, parent of Kevelt. Specifically, we issued 1,373,036 shares of common stock for the conversion of notes held by Pharmsynthez plus interest accrued thereon, and 3,045,455 shares of common stock in consideration for our purchase of certain intellectual property rights from Pharmsynthez. Pursuant to the Kevelt APA, we also issued certain management warrants to certain members of management of Pharmsynthez. Such warrants may be exercised at any time on or after March 31, 2016 through the five-year anniversary of the issuance thereof. |
| 10. | On July 1, 2016, we issued a warrant to purchase 37,369 shares of our common stock to our Chief Executive Officer, Mr. M. Scott Maguire, for his deferment of salary. The initial exercise price for the purchase of the warrant is $6.60 per share with a term of five years from the grant date. This warrant was fully vested on the date of grant.
| |
|
11. |
During the third quarter of 2016, we issued $1.0 million of convertible promissory notes to Pharmsynthez. | |
| 12. | On September 23, 2016, SynBio, LLC exchanged 970,000 shares of common stock in the Company for an equal number of shares of Series A Preferred Stock. |
We deemed the offers, sales and issuances of the securities described in paragraphs 1 through 8, 11 and 12 above to be exempt from registration under the Securities Act, in reliance on Section 4(a)(2) of the Securities Act, including Regulation D and Rule 506 promulgated thereunder, regarding transactions by an issuer not involving a public offering. All purchasers of securities in transactions exempt from registration pursuant to Regulation D represented to us that they were accredited investors and were acquiring the shares for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
We deemed the grants of stock options described in paragraph 9 and the issuance of a warrant described in paragraph 10 as exempt pursuant to Section 4(a)(2) of the Securities Act or to be exempt from registration under the Securities Act in reliance on Rule 701 of the Securities Act as offers and sales of securities under compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships, to information about us.
All certificates representing the securities issued in the transactions described in this Item 15 included appropriate legends setting forth that the securities had not been offered or sold pursuant to a registration statement and describing the applicable restrictions on transfer of the securities. There were no underwriters employed in connection with any of the transactions set forth in this Item 15.
Item 16. Exhibits and Financial Statement Schedules.
| (a) | Exhibits. The exhibits to the registration statement are listed in the Exhibit Index to this registration statement. | |
| (b) | Financial Statements Schedules. Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto. |
| II-3 |
Item 17. Undertakings.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended (the Act), may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue. The Registrant hereby undertakes that:
| (a) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | To include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement. |
| (b) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (c) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (d) | That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| (e) | The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant's annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan's annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| II-4 |
| (f) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue. |
| (g) | For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (h) | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| II-5 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Lexington, Commonwealth of Massachusetts, on October 21, 2016.
| XENETIC BIOSCIENCES, INC. | ||
| By: | /s/ M. Scott Maguire | |
| M. Scott Maguire | ||
| Chief Executive Officer and President | ||
| II-6 |
POWER OF ATTORNEY AND SIGNATURES
We, the undersigned officers and directors of Xenetic Biosciences, Inc., hereby severally constitute and appoint M. Scott Maguire, our true and lawful attorney, with full power, to sign for us in our names in the capacities indicated below, all amendments to this report, and generally to do all things in our names and on our behalf in such capacities to enable Xenetic Biosciences, Inc. to comply with the provisions of the Securities Exchange Act of 1934, as amended, and all requirements of the Securities and Exchange Commission.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement and Power of Attorney has been signed by the following person in the capacities and on the date indicated.
| Name | Title | Date | ||
| /s/ M. Scott Maguire | President, Chief Executive Officer | October 21, 2016 | ||
| M. Scott Maguire | and Director (Principal Executive Officer and | |||
| Principal Financial Officer) | ||||
| * | Director | October 21, 2016 | ||
| Firdaus Jal Dastoor FCS | ||||
| * | Director | October 21, 2016 | ||
| Jeffrey F. Eisenberg | ||||
| * | Director | October 21, 2016 | ||
| Darlene Deptula-Hicks | ||||
| * | Director | October 21, 2016 | ||
| Dr. Roger Kornberg | ||||
| * | Director | October 21, 2016 | ||
| Roman Knyazev | ||||
| *By: | /s/ M. Scott Maguire | October 21, 2016 | |||
| M. Scott Maguire | |||||
| Attorney-in-Fact | |||||
| II-7 |
EXHIBIT INDEX
| Exhibit No. | Exhibit Index | Form | Incorporated by Reference Date | Number | Filed Herewith |
| 1.1 | Form of Underwriting Agreement | S-1 | 10/11/2016 | 1.1 | |
| 2.1 | Scheme of Arrangement (court order) | 8-K | 01/29/2014 | 2.1 | |
| 3.1 | Articles of Incorporation | S-1 | 11/21/2011 | 3.1 | |
| 3.2 | Certificate of Amendment to Articles of Incorporation | 8-K | 02/12/2013 | 3.1 | |
| 3.3 | Certificate of Amendment to Articles of Incorporation | 8-K | 02/27/2013 | 3.1 | |
| 3.4 | Certificate of Amendment to Articles of Incorporation | 10-Q | 01/10/2014 | 3.1 | |
| 3.5 | Certificate of Change Pursuant to NRS 78.209 | 10-Q | 01/10/2014 | 3.2 | |
| 3.6 | Certificate of Amendment to Articles of Incorporation | 8-K | 09/30/2015 | 3.1 | |
| 3.7 | Bylaws | S-1 | 11/21/2011 | 3.2 | |
| 3.8 | Certificate of Designation of Preferences, Rights and Limitations of Series A Preferred Stock | 8-K | 09/23/2016 | 3.1 | |
| 3.9 | Form of Certificate of Designation of Preferences, Rights and Limitations of Series B Preferred Stock | S-1 | 10/11/2016 | 3.9 | |
| 4.1 | Form of Common Stock Certificate of the Registrant | S-1 | 07/14/2016 | 4.1 | |
| 5.1 | Legal Opinion | X | |||
| 10.1 | Possible Offer for Xenetic Biosciences plc by General Sales & Leasing, Inc., dated October 21, 2013 | 8-K | 10/21/2013 | 9.1 | |
| 10.2 | Recommended Acquisition of Xenetic Biosciences plc by General Sales & Leasing, Inc. including Scheme of Arrangement | 8-K and 8-K/A | 11/25/2013 | 9.1 | |
| 10.3 | Announcement of Recommended Offer by General Sales and Leasing, Inc. for shares of Xenetic Biosciences plc, dated November 12, 2013 | 8-K | 11/25/2013 | 9.2 | |
| 10.4 | Agreement of Conveyance, Transfer and Assignment of Subsidiaries and Assumption of Obligations dated November 12, 2013 between General Sales Inc., Leasing, Inc., Oxbridge Technology Partners, SA, Shift It Media Company and General Aircraft, Inc. | 10-K | 11/27/2013 | 9.3 | |
| 10.5† | Form of Rules of the Lipoxen plc Unapproved Share Option Plan dated July 18, 2000 (as amended by a resolution of the board of directors of Lipoxen plc passed on March 14, 2006) | 10-K | 04/15/2014 | 10.5 | |
| 10.6† | Form of Xenetic Biosciences plc 2007 Share Option Scheme and US Addendum (as established in 2007 and by resolution of shareholders in 2010 and awarded by board resolution in 2012) | 10-K | 04/15/2014 | 10.6 | |
| 10.7† | Form of Xenetic Biosciences, Inc. Equity Incentive Plan, effective January 23, 2014 | 10-K | 04/15/2014 | 10.7 | |
| 10.8 | Master Clinical Research Services Agreement between Novotech Pty Limited and Xenetic Biosciences plc dated Feb. 6, 2013 | 10-K | 04/15/2014 | 10.17 | |
| 10.9† | Employment Agreement, dated November 3, 2009, between Lipoxen plc and M. Scott Maguire | 10-K/A | 02/18/2015 | 10.01 | |
| 10.10 | Form of Lease for Ledgemont Research Center, Lexington, Massachusetts dated August 1, 2013 between One Ledgemont LLC and Xenetic Bioscience, Inc. | 10-K/A | 02/18/2015 | 10.03 | |
| 10.11 | Stock Purchase Agreement, dated January 29, 2014, between Xenetic Biosciences, Inc. and Baxter Healthcare SA | 10-K/A | 02/18/2015 | 10.08 | |
| 10.12 | Stock Purchase Agreement Amendment No. 1, dated February 14, 2014, between Xenetic Biosciences, Inc. and Baxter Healthcare SA | 10-K/A | 02/18/2015 | 10.09 |
| II-8 |
| Exhibit No. | Exhibit Index | Form | Incorporated by Reference Date | Number | Filed Herewith |
| 10.13 | Exclusive Research, Development and License Agreement, dated August 15, 2005, between Lipoxen Technologies Limited, Baxter Healthcare SA and Baxter Healthcare Corporation | 10-K/A | 02/18/2015 | 10.10 | |
| 10.14 | Letter Agreement, dated December 11, 2006, between Lipoxen Technologies Limited, Baxter Healthcare SA, Baxter Healthcare Corporation and Serum Institute of India Limited | 10-K/A | 02/18/2015 | 10.11 | |
| 10.15 | Amendment to the Exclusive Research, Development and License Agreement, dated December 13, 2006, between Lipoxen Technologies Limited, Baxter Healthcare SA and Baxter Healthcare Corporation | 10-K/A | 02/18/2015 | 10.12 | |
| 10.16 | Second Amendment to the Exclusive Research, Development and License Agreement, dated May 28, 2009, between Lipoxen Technologies Limited, Baxter Healthcare SA and Baxter Healthcare Corporation | 10-K/A | 02/18/2015 | 10.13 | |
| 10.17 | Amendment Number Four to the Exclusive Research, Development and License Agreement, dated August 10, 2010, between Lipoxen Technologies Ltd., Baxter Healthcare SA and Baxter Healthcare Corporation | 10-K/A | 02/18/2015 | 10.14 | |
| 10.18 | Amendment Number Five to the Exclusive Research, Development and License Agreement, dated September 15, 2010, between Lipoxen Technologies Ltd., Baxter Healthcare SA and Baxter Healthcare Corporation | 10-K/A | 02/18/2015 | 10.15 | |
| 10.19 | Form of Sixth Amendment to the Exclusive Research, Development and License Agreement, dated January 29, 2014, between Lipoxen Technologies Limited, Baxter Healthcare SA and Baxter Healthcare Corporation | 10-K/A | 02/18/2015 | 10.16 | |
| 10.20 | Agreement on Co-Development and the Terms of Exclusive License dated August 4, 2011 between Lipoxen plc, Lipoxen Technologies LTD and SynBio LLC | 10-K/A | 02/18/2015 | 10.18 | |
| 10.21 | Subscription Agreement in respect of ordinary shares in the capital of Lipoxen plc dated August 4, 2011 between SynBio LLC and Lipoxen plc | 10-K/A | 02/18/2015 | 10.19 | |
| 10.22 | Collaboration, Licence and Development Agreement, dated November 11, 2009, between Pharmasynthez ZAO and Lipoxen Technologies Ltd. | 10-K/A | 02/18/2015 | 10.20 | |
| 10.23 | Exclusive Patent and Know How Licence and Manufacturing Agreement, dated August 4, 2011, between Lipoxen plc, Lipoxen Technologies Ltd and Serum Institute of India Limited | 10-K/A | 02/18/2015 | 10.21 | |
| 10.24† | Employment Agreement, dated April 30, 2012, between Xenetic Bioscience, Inc. and Dr. Henry Hoppe IV. | 10-K/A | 02/18/2015 | 10.23 | |
| 10.25 | Intellectual Property Assignment between Dmitry Genkin, FDS Pharma, Lipoxen Technologies Limited and Xenetic Biosciences Inc. | 10-K | 04/15/2015 | 10.1 | |
| 10.26 | SynBio LLC Warrant to Purchase Common Stock of Xenetic Bioscience, Incorporated | 10-K | 04/15/2015 | 10.2 | |
| 10.27 | Serum Institute of India Limited Warrant to Purchase Common Stock of Xenetic Bioscience, Incorporated | 10-K | 04/15/2015 | 10.03 | |
| 10.28 | Firdaus Jal Dastoor Warrant to Purchase Common Stock of Xenetic Bioscience, Incorporated | 10K | 04/15/2015 | 10.4 | |
| 10.29 | Securities Purchase Agreement, dated May 2015, between Xenetic Bioscience, Inc. and OJSC Pharmsynthez | 8-K | 07/08/2015 | 10.1 | |
| 10.30 | Ten Percent (10%) Senior Secured Collateralized Convertible Promissory Note, dated July 1, 2015, between Xenetic Bioscience, Inc. and OJSC Pharmsynthez | 8-K | 07/08/2015 | 10.2 |
| II-9 |
| Exhibit No. | Exhibit Index | Form | Incorporated by Reference Date | Number | Filed Herewith |
| 10.31 | Registration Rights Agreement, dated July 1, 2015, between Xenetic Bioscience, Inc. and OJSC Pharmsynthez | 8-K | 07/08/2015 | 10.3 | |
| 10.32 | Security Agreement dated July 1, 2015, between Xenetic Bioscience, Inc. and OJSC Pharmsynthez | 8-K | 07/08/2015 | 10.4 | |
| 10.33 | Subsidiary Guarantee dated July 1, 2015, between Xenetic Bioscience, Inc. and OJSC Pharmsynthez | 8-K | 07/08/2015 | 10.5 | |
| 10.34 | Common Stock Purchase Warrant, dated July 1, 2015 | 8-K | 07/08/2015 | 10.6 | |
| 10.35 | Form of Assignment and Assumption Agreement | 8-K | 07/08/2015 | 10.7 | |
| 10.36 | Settlement Agreement, dated August 27, 2015, between Xenetic Biosciences (UK) Limited, Xenetic Biosciences, Inc., Lipoxen Technologies Limited and Colin Hill | 8-K | 09/02/2015 | 10.1 | |
| 10.37 | Form of Asset Purchase Agreement, dated as of November 13, 2015, by and among Xenetic Biosciences, Inc., Lipoxen Technologies, LTD, a U.K. corporation, AS Kevelt, an Estonian company and OJSC Pharmsynthez | 8-K | 11/16/2015 | 10.1 | |
| 10.38 | Form of Ten Percent (10%) Senior Secured Convertible Promissory Note | 8-K | 11/16/2015 | 10.2 | |
| 10.39 | Form of Common Stock Purchase Warrant | 8-K | 11/16/2015 | 10.3 | |
| 10.40 | Form of Common Stock Purchase Warrant | 8-K | 11/16/2015 | 10.4 | |
| 10.41 | Form of Amended and Restated Ten Percent (10%) Senior Secured Convertible Promissory Note | 8K | 11/16/2015 | 10.5 | |
| 10.42 | Form of Amended and Restated Common Stock Purchase Warrant | 8-K | 11/16/2015 | 10.6 | |
| 10.43 | Form of First Amendment to Securities Purchase Agreement | 8-K | 11/16/2015 | 10.7 | |
| 10.44 | Form of First Amendment to Registration Rights Agreement | 8-K | 11/16/2015 | 10.8 | |
| 10.45 | Form of First Amendment to Security Agreement | 8-K | 11/16/2015 | 10.9 | |
| 10.46 | Form of First Amendment to Subsidiary Guarantee | 8-K | 11/16/2015 | 10.10 | |
| 10.47 | Form of Transition, Services and Resupply Agreement by and among Xenetic Bioscience, Inc., AS Kevelt and OJSC Pharmsynthez | 8-K | 11/16/2015 | 10.11 | |
| 10.48 | Letter Agreement re. Appointment of Non – Employee, Independent Director of Xenetic Biosciences, Inc. for Roger D. Kornberg dated February 2016 | 8-K | 02/29/2016 | 10.1 | |
| 10.49 | Deferred Salary Security Agreement with Mr. Maguire | 8-K | 07/06/2016 | 10.1 | |
| 10.50 | Form of Ten Percent (10%) Junior Secured Convertible Promissory Note – Due Deferral End Date | 8-K | 07/06/2016 | 10.2 | |
| 10.51 | Form of Common Stock Purchase Warrant | 8-K | 07/06/2016 | 10.3 | |
| 10.52 | Letter Agreement re. Appointment of Non – Employee, Independent Director of Xenetic Biosciences, Inc. for Jeffrey F. Eisenberg dated July 8, 2016 | 8-K | 07/17/2016 | 10.1 | |
| 10.53 | Form of Common Stock Purchase Warrant | S-1 | 10/11/2016 | 10.53 | |
| 21.1 | List of Subsidiaries | S-1 | 06/24/2016 | 21.1 | |
| 23.1 | Consent of Marcum LLP | X | |||
| 23.2 | Consent of Ernst & Young LLP | X | |||
| 23.3 | Consent of Counsel (included in Exhibit 5.1) | X | |||
| 24.1 | Power of Attorney (included on signature page) | S-1 | 05/09/2016 | 24.1 | |
| 101 | XBRL (eXtensible Business Reporting Language) | S-1/A | 06/24/2016 | 101 |
| * | To be filed by amendment | |
| † | Indicates a management contract or any compensatory plan, contract or arrangement. |
| II-10 |