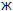 ” Krasnovo Kursanta St., St. Petersburg, 197 110, Russia.
” Krasnovo Kursanta St., St. Petersburg, 197 110, Russia.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 13D
Under the Securities Exchange Act of 1934
Xenetic Biosciences, Inc.
(Name of Issuer)
COMMON STOCK, $0.001 PER SHARE PAR VALUE
(Title of Class of Securities)
984015 206
(CUSIP Number)
Neal Aizenstein, Esq.
DLA Piper LLP (US)
444 West Lake Street, Suite 900, Chicago, Illinois 60606-0089
(Name, Address and Telephone Number of Person Authorized to Receive Notices and Communications)
November 1, 2016
(Date of Event Which Requires Filing of this Statement)
If the filing person has previously filed a statement on Schedule 13G to report the acquisition that is the subject of this Schedule 13D, and is filing this schedule because of Rule 13d-1(e), Rule 13d-1(f) or Rule 13d-1(g), check the following box. ☐
| * | The remainder of this cover page shall be filled out for a reporting person’s initial filing on this form with respect to the subject class of securities, and for any subsequent amendment containing information which would alter the disclosures provided in a prior cover page. |
The information required in the remainder of this cover page shall not be deemed to be “filed” for the purpose of Section 18 of the Securities Exchange Act of 1934 (“Act”) or otherwise subject to the liabilities of that section of the Act but shall be subject to all other provisions of the Act (however, see the Notes).
| CUSIP No. 984015 206 | SCHEDULE 13D |
| 1. | Names of Reporting Persons:
PJSC Pharmsynthez | |||||
| 2. | Check the Appropriate Box if a Member of a Group (See Instructions) (a) ☒ (b) ☐
| |||||
| 3. | SEC Use Only:
| |||||
| 4. | Source of Funds (See Instruction):
OO, WC (see item 6) | |||||
| 5. | Check if Disclosure of Legal Proceedings is Required Pursuant to Items 2(d) or 2(e): ☐
| |||||
| 6. | Citizenship or Place of Organization:
Russia | |||||
| Number of Shares Beneficially by Owned by Each Reporting Person with:
|
7. | Sole Voting Power:
7,059,646 SHARES | ||||
| 8. | Shared Voting Power:
821,565 SHARES | |||||
| 9. | Sole Dispositive Power:
7,059,646 SHARES | |||||
| 10. | Shared Dispositive Power:
821,565 SHARES | |||||
| 11. |
Aggregate Amount Beneficially Owned by Each Reporting Person:
7,881,211 SHARES1 The total beneficial ownership consists of 5,366,053 shares of common stock owned directly and indirectly through Pharmsynthez’s wholly-owned subsidiary SynBio, including 145,455 held in escrow, 1,454,545 shares issuable upon conversion of Series B Preferred Stock and 1,060,613 shares issuable upon exercise of warrants that are exercisable within 60 days of July 12, 2017. | |||||
| 12. | Check if the Aggregate Amount in Row (11) Excludes Certain Shares (See Instructions): ☒2
| |||||
| 13. | Percent of Class Represented by Amount in Row (11):
70.2% | |||||
| 14. | Type of Reporting Person (See Instructions):
CO | |||||
| 1 | The Group is deemed to beneficially own an aggregate of 7,936,771 Shares, or 70.4% of the outstanding Shares. |
| 2 | Excludes (a) 970,000 shares of Series A Preferred Stock owned by Pharmsynthez’s subsidiary, LLC SynBio, which are convertible into common stock upon 61 days prior notice and (b) 1,454,545 Class B Warrants owned by Pharmsynthez which may not be exercisable within 60 days of July 12, 2017. |
- 2 -
| CUSIP No. 984015 206 | SCHEDULE 13D |
| 1. | Names of Reporting Persons:
LLC SynBio | |||||
| 2. | Check the Appropriate Box if a Member of a Group (See Instructions) (a) ☒ (b) ☐
| |||||
| 3. | SEC Use Only:
| |||||
| 4. | Source of Funds (See Instruction):
OO, WC ( See item 6) | |||||
| 5. | Check if Disclosure of Legal Proceedings is Required Pursuant to Items 2(d) or 2(e): ☐
| |||||
| 6. | Citizenship or Place of Organization:
Russia | |||||
| Number of Shares Beneficially by Owned by Each Reporting Person with:
|
7. | Sole Voting Power:
0 SHARES | ||||
| 8. | Shared Voting Power:
821,565 SHARES | |||||
| 9. | Sole Dispositive Power:
0 SHARES | |||||
| 10. | Shared Dispositive Power:
821,565 SHARES | |||||
| 11. |
Aggregate Amount Beneficially Owned by Each Reporting Person:
821,565 SHARES3 | |||||
| 12. | Check if the Aggregate Amount in Row (11) Excludes Certain Shares (See Instructions): ☒4
| |||||
| 13. | Percent of Class Represented by Amount in Row (11):
9.4 % | |||||
| 14. | Type of Reporting Person (See Instructions):
CO | |||||
| 3 | The Group is deemed to beneficially own an aggregate of 7,936,771 Shares, or 70.4% of the outstanding Shares. |
| 4 | Excludes 970,000 shares of Series A Preferred Stock which are convertible into common stock upon 61 days prior notice and warrants to acquire 204,394 shares of common stock. |
- 3 -
| CUSIP No. 984015 206 | SCHEDULE 13D |
| 1. | Names of Reporting Persons:
Dmitry Genkin | |||||
| 2. | Check the Appropriate Box if a Member of a Group (See Instructions) (a) ☒ (b) ☐
| |||||
| 3. | SEC Use Only:
| |||||
| 4. | Source of Funds (See Instruction):
OO (see item 6) | |||||
| 5. | Check if Disclosure of Legal Proceedings is Required Pursuant to Items 2(d) or 2(e): ☐
| |||||
| 6. | Citizenship or Place of Organization:
Russia | |||||
| Number of Shares Beneficially by Owned by Each Reporting Person with:
|
7. | Sole Voting Power:
55,557 SHARES | ||||
| 8. | Shared Voting Power:
SHARES | |||||
| 9. | Sole Dispositive Power:
55,557 SHARES | |||||
| 10. | Shared Dispositive Power:
| |||||
| 11. |
Aggregate Amount Beneficially Owned by Each Reporting Person:
55,557 SHARES5 The total beneficial ownership consists of 25,253 shares of common stock issuable upon exercise of stock options that are exercisable within 60 days of July 12, 2017 and 30,304 shares of common stock issuable upon exercise of warrants that are exercisable within 60 days of July 12, 2017. | |||||
| 12. | Check if the Aggregate Amount in Row (11) Excludes Certain Shares (See Instructions): ☐
| |||||
| 13. | Percent of Class Represented by Amount in Row (11):
0.6 % | |||||
| 14. | Type of Reporting Person (See Instructions):
IN | |||||
| 5 | The Group is deemed to beneficially own an aggregate of 7,936,771 Shares, or 70.4% of the outstanding Shares. |
- 4 -
| ITEM 1. | SECURITY AND ISSUER. |
This Statement on Schedule 13D relates to the common stock, par value $0.001 per share (the “Shares”), of Xenetic Biosciences, Inc. a Nevada Corporation (the “Issuer”), and is being filed by PJSC Pharmsynthez, LLC SynBio and Dmitry Genkin (the “Reporting Persons”). The Issuer’s current principal executive offices are located at 99 Hayden Ave, Suite 230, Lexington, MA 02421.
| ITEM 2. | IDENTITY AND BACKGROUND |
The persons filing this statement and the persons enumerated in Instruction C of Schedule 13D and, where applicable, their respective places of organization, general partners, directors, executive officers and controlling persons, and the information regarding them, are as follows:
| (a) | PJSC Pharmsynthez |
LLC SynBio
Dmitry Genkin
(collectively, the “Reporting Persons”)
| (b) | The business addresses of the Reporting Persons are: |
The business address of PJSC
Pharmsynthez, LLC SynBio and Dmitry Genkin is 25 liter “
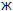 ” Krasnovo Kursanta St., St. Petersburg, 197 110, Russia.
” Krasnovo Kursanta St., St. Petersburg, 197 110, Russia.
| (c) | Present principal occupation or employment of the Reporting Persons and the name, principal business and address of any corporation or other organization in which such employment is conducted: |
Pharmsynthez is an innovative pharmaceutical company developing new medications, technologies of drugs for organ-specific delivery and
innovative methods of manufacture of pharmaceutical ingredients. The address of Pharmsynthez’s principal business and principal office is 25 liter “
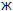 ” Krasnovo Kursanta St., St. Petersburg, 197 110, Russia.
” Krasnovo Kursanta St., St. Petersburg, 197 110, Russia.
SynBio brings together Russian and international technology and know-how for the purpose of creating innovative new medicines. The address of SynBio’s principal business and principal office is 55/1 bild 2, Leninskii prosp., Moscow, Russia. SynBio was acquired by Pharmsynthez in 2017.
Dmitry Genkin is principally employed as Chairman of Pharmsynthez. His business address is 25 liter “
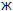 ” Krasnovo Kursanta St., St. Petersburg, 197 110, Russia.
” Krasnovo Kursanta St., St. Petersburg, 197 110, Russia.
Exhibit C hereto, which is incorporated herein by reference, sets forth the name, business address, present principal occupation of employment (and the name, principal business and address of any corporation or other organization in which such employment is conducted) and the citizenship of the directors, executive officers and control persons of Pharmsynthez and SynBio.
| (d) | During the last five years, none of the Reporting Persons and, to the best knowledge of the Reporting Persons, none of the Persons listed on Exhibit C hereto, has been convicted in a criminal proceeding (excluding traffic violations or similar misdemeanors). |
| (e) | During the last five years, none of the Reporting Persons and, to the best knowledge of the Reporting Persons, none of the Persons listed on Exhibit C hereto, was a party to a civil proceeding of a judicial or administrative body of competent jurisdiction and as a result of such proceeding was or is subject to a judgment, decree or final order enjoining future violations of, or prohibiting or mandating activities subject to, federal or state securities laws or finding any violation with respect to such laws. |
| (f) | For citizenship of the Reporting Persons, see Item 4 of the cover sheet for each Reporting Person. |
- 5 -
| ITEM 3. | SOURCE AND AMOUNT OF FUNDS OR OTHER CONSIDERATION. |
The Shares to which this statement relates were acquired pursuant to transactions with the Issuer. Refer to Item 6 below for a description of these transactions.
| ITEM 4. | PURPOSE OF TRANSACTION |
The Reporting Persons intend to evaluate their investment in the Issuer on a continual basis. The Reporting Persons may discuss the prospects and affairs of the Issuer, and the status of the Reporting Person’s investment in the Issuer at any time and from time to time, with the board of directors of the Issuer or any of the Issuer’s subsidiaries or the executive officers of the Issuer or the Issuer’s subsidiaries. The Reporting Persons may discuss ideas that, if effected, could result in a corporate transaction involving the Issuer, changes in the board of directors or management of the Issuer or other matters. In this regard, Pharmsynthez intends to discuss with the board of directors of the Issuer the composition of the board of directors and management of the Issuer.
Except as provided above, the Reporting Person does not have any current plans or proposals which would relate to or would result in:
| (a) | the acquisition by any person of additional securities of the Issuer, or the disposition of securities of the Issuer; |
| (b) | any extraordinary corporate transaction, such as a merger, reorganization or liquidation, involving the Issuer or any of its subsidiaries; |
| (c) | a sale or transfer of a material amount of the assets of the Issuer or any of its subsidiaries; |
| (d) | any change in the present board of directors or management of the Issuer, including any plans or proposals to change the number or term of directors or to fill any existing vacancies on the board; |
| (e) | any material change in the present capitalization or dividend policy of the Issuer; |
| (f) | any other material change in the Issuer’s business or corporate structure including, but not limited to, if the Issuer is a registered closed-end investment company, any plans or proposals to make any changes in its investment policy for which a vote is required by Section 13 of the Investment Company Act of 1940; |
| (g) | changes in the Issuer’s charter, bylaws or instruments corresponding thereto or other actions which may impede acquisition of control of the Issuer by any person; |
| (h) | causing a class of securities of the Issuer to be delisted from a national securities exchange or to cease to be authorized to be quoted in an inter-dealer quotation system of a registered national securities association; |
| (i) | a class of equity securities of the Issuer becoming eligible for termination of registration pursuant to Section 12(g)(4) of the Act; or |
| (j) | any action similar to any of those enumerated above. |
| ITEM 5. | INTEREST IN SECURITIES OF THE ISSUER. |
The beneficial ownership of the Shares by each Reporting Person at the date hereof is reflected on that Reporting Person’s cover page. Certain determinations of beneficial ownership with respect to shares of preferred stock of the Issuer and warrants with performance vesting are based on publicly available information and the Reporting Persons reserve all rights under the underlying preferred stock and warrants with respect to the exercise thereof and otherwise.
Pursuant to Rule 13d-4 of the Exchange Act, to the extent permitted by law, each of the Reporting Persons expressly declares that that filing of this Schedule 13D (and any amendment thereto) shall not be construed as an admission
- 6 -
that any such person is, for the purposes of Section 13(d) and/or Section 13(g) of the Exchange Act or otherwise, (i) the beneficial owner of any Shares held by any other person, or (ii) the beneficial owner of any Shares held or beneficially owned by any member of the Group other than the Reporting Person.
The filing of this Schedule 13D (and any amendment thereto) by each of the Reporting Persons shall not, to the extent permitted by law, be considered an admission that such Reporting Person, for the purposes of Section 13(d) of the Exchange Act, is the beneficial owner of Shares in which such Reporting Person does not have a pecuniary interest.
SynBio is a wholly-owned subsidiary of Pharmsynthez. Pharmsynthez may be deemed to have shared power to vote or direct the vote of (and the shared power to dispose or direct the disposition) of the Shares owned by SynBio and therefore, Pharmsynthez may deemed to be the beneficial owner of such Shares.
Mr. Genkin, by virtue of his position with Pharmsynthez, may be deemed to have the shared power to vote or direct the vote of (and the shared power to dispose or direct the disposition of) the Shares beneficially owned by Pharmsynthez and therefore, each may be deemed to be the beneficial owner of such Shares. Mr. Genkin disclaims beneficial ownership of such Shares.
| ITEM 6. | CONTRACTS, ARRANGEMENTS, UNDERSTANDINGS OR RELATIONSHIPS WITH RESPECT TO SECURITIES OF THE ISSUER. |
Other than as described in this Schedule 13D, the Reporting Persons have no contracts, arrangements, understandings or relationships with any other person with respect to any securities of the Issuer.
PJSC Pharmsynthez
In November 2009, the Issuer entered into a collaborative research and development license agreement with Pharmsynthez (the “Pharmsynthez Arrangement”) pursuant to which the Issuer granted an exclusive license to Pharmsynthez to develop, commercialize and market six drug candidates based on the Issuer’s PolyXen and ImuXen technology in certain territories. In exchange, Pharmsynthez granted the Issuer an exclusive license to use any pre-clinical and clinical data developed by Pharmsynthez, within the scope of the Pharmsynthez Arrangement, and to engage in further research, development and commercialization of drug candidates outside of certain territories at the Issuer’s own expense.
In July 2015, the Issuer entered into a Securities Purchase Agreement with Pharmsynthez providing for the issuance of $3.0 million of convertible promissory note and warrants to purchase an aggregate of 606,062 Shares. These warrants have a five year term and are exercisable at the lesser of $6.60 per share and 120% of the price per share in the Issuer’s next capital sale of at least $7 million (the “Exercise Price”).
In November 2015, the Issuer entered into an Asset Purchase Agreement (the “APA”) with Pharmsynthez. The APA provided for the transfer to the Issuer of certain intellectual property rights with respect to the immunomodulatory drug candidate XBIO-101. In April 2016, the Issuer completed the purchase of the intellectual property rights associated with the APA and issued 3,045,455 Shares pursuant to the APA.
The APA also provided for the Issuer’s issuance of certain convertible promissory notes and warrants to purchase Shares. During the quarter ended March 31, 2016, the Issuer issued $3.5 million of convertible promissory notes to Pharmsynthez receiving $3.5 million in cash proceeds. In April 2016, Pharmsynthez converted all of its convertible promissory notes (both the July 2015 note and the Q1 2016 notes) and associated interest into 1,373,036 Shares. As such, the Issuer issued to Pharmsynthez 1,373,036 Shares in connection with conversion of the convertible notes, which amount, together with the 3,045,455 Shares in connection with the closing of the APA, resulted in an aggregate of 4,418,491 new Shares being issued to Pharmsynthez.
In connection with the Issuer’s issuance of the convertible promissory notes in March 2016, the Issuer issued to Pharmsynthez warrants to purchase 353,540 Shares at the Exercise Price.
- 7 -
During the quarter ended September 30, 2016, the Issuer issued $1.0 million of convertible promissory notes to Pharmsynthez, receiving $1.0 million in cash proceeds of which $0.5 million of the notes was applied toward the Issuer’s November 2016 offering. In connection with the Issuer’s issue of the convertible promissory warrants, the Issuer issued to Pharmsynthez warrants to purchase 50,505 Shares at the Exercise Price.
On November 1, 2016, Pharmsynthez purchased 1,454,545 Shares of Convertible Series B Preferred Stock [and 1,454,545 Class B Warrants with an exercise price of $4.00 per share in the Issuer’s initial public offering for $6.0 million.
LLC SynBio
In August 2011, the Issuer entered into a Co-Development Agreement with SynBio, which is still in effect, pursuant to which the Issuer granted an exclusive license to SynBio to develop pharmaceutical products within Russia and the CIS using certain molecule(s) based on SynBio’s technology and the Issuer’s proprietary technologies: PolyXen, OncoHist and ImuXen. In return, SynBio granted the Issuer an exclusive license to use the pre-clinical and clinical data generated by SynBio in certain agreed upon products and engage in the development of commercial drug candidates.
The Co-Development Agreement provides for the sale of certain research supplies between each party to the agreement. For the years ended December 31, 2016 and 2015, the Issuer did not recognize any supply service revenues from sales to SynBio about the Co-Development Agreement.
Concurrent with entering into the Co-Development Agreement, the Issuer entered into a stock subscription agreement with SynBio pursuant to which the Issuer sold SynBio approximately 1.8 million Shares.
In furtherance of the Issuer’s co-development clinical objectives, on December 31, 2014 the Issuer granted SynBio a warrant to purchase 204,394 Shares that contain vesting triggers based on the achievement by SynBio of certain clinical development objectives within specific timeframes with an exercise price of $25.41 per share (the “SynBio 2014 Warrant”). The SynBio 2014 Warrant expires on December 30, 2019.
On September 23, 2016, SynBio exchanged 970,000 Shares for an equal number of Shares of Series A Preferred Stock.
Effective October 26, 2016, the Issuer entered into a voting agreement with SynBio whereby SynBio agreed to vote their Shares in the same proportion as the Shares held by non-affiliates on all matters subject to stockholder approval for a period of one year. A copy of this voting agreement is attached hereto as Exhibit B.
In May 2011, the Issuer received a short-term unsecured loan facility of up to $1.7 million from SynBio, of which $0 was outstanding as of December 31, 2016. In connection with the APA, the Issuer made a series of payments during 2016 totaling $323,640 to creditors. Pursuant to the APA such payments are considered direct offsets to the loan with SynBio. The loan had an interest rate of 8.04% per annum as of the date of grant, with interest payable upon repayment of the loan, which was to be seven months after the closing date of the loan. In December 2016, the Issuer entered into an agreement with SynBio and Pharmsynthez, which settled all amounts owed by the Issuer on the SynBio loan; for services provided to the Issuer in connection with the XBIO-101 Phase 2 project; and the purchase of drug candidate supply sufficient to meet the needs of the XBIO-101 Phase 2 clinical trial. Pursuant to the agreement, the Issuer transferred $620,387 to the counterparties. The Issuer repaid the remainder of the loan facility with proceeds from the Issuer’s November 1, 2016 offering.
Dmitry Genkin
Dmitry Genkin served as a non-employee director of the Issuer from September 2015 until May 2016 and received stock options to purchase an aggregate of 37,879 Shares with an exercise price of $4.59 per share which vest equally over a three year period (25,253 of which are exercisable within 60 days of July 12, 2017). In addition, Mr. Genkin holds a warrant to purchase 30,304 Shares at $13.86 per share. These warrants are fully vested and expire in November 2020.
- 8 -
The Issuer previously had an agreement with Dmitry Genkin under which he was entitled to an annual fee of £750 paid in quarterly installments for his services as director of the Issuer. During 2016 and 2015, Mr. Genkin was paid approximately $1,000 annually in respect of director services provided in each year. In addition, Dmitry Genkin previously provided research and consulting services to the Issuer, for which he was paid approximately $129,000 and $73,000 during the years ended December 31, 2016 and 2015, respectively. In May 2016, Mr. Genkin resigned from the Board of Directors of the Issuer.
| ITEM 7. | MATERIAL TO BE FILED AS EXHIBITS. |
| Exhibit A - | Agreement Regarding Joint Filing of Statement on Schedule 13D or 13G | |
| Exhibit B - | Shareholder Voting Agreement dated October 26, 2016 between Xenetic Biosciences, Inc. and LLC SynBio | |
| Exhibit C - | Certain Information Regarding Directors and Executive Officers of Pharmsynthez and SynBio | |
- 9 -
SIGNATURE
After reasonable inquiry and to the best of my knowledge and belief, I certify that the information set forth in this statement is true, complete and correct.
Date: July 12, 2017
| PJSC PHARMSYNTHEZ | LLC SYNBIO | |||||||
| By: | /s/ Petr (Vladimirovich) Kruglyakov |
By: | /s/ Sergey (Sergeevich) Avtushenko | |||||
| Petr (Vladimirovich) Kruglyakov | Sergey (Sergeevich) Avtushenko | |||||||
| /s/ Dmitry Genkin |
||||||||
| Dmitry Genkin | ||||||||